HQ Team
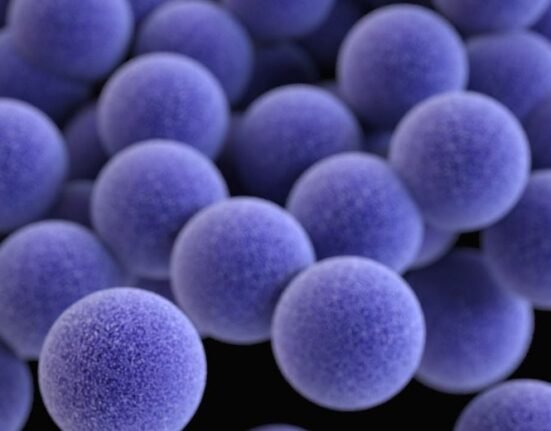
April 15, 2024: Superbugs are held responsible for most hospital-acquired infections, however, research delving into the genetic data of bacteria causing these infections is surprising: the majority of healthcare-associated infections stem from harmless bacteria residing on patients’ bodies before they even enter the hospital environment.
Recent studies comparing bacteria in the microbiome – the communities of microorganisms inhabiting our noses, skin, and other body areas – with those causing infections like pneumonia, diarrhoea, bloodstream infections, and surgical site infections have shed light on this belief. Contrary to conventional beliefs, innocuous bacteria present on our bodies become perpetrators of serious infections during illness.
Innocuous bacteria
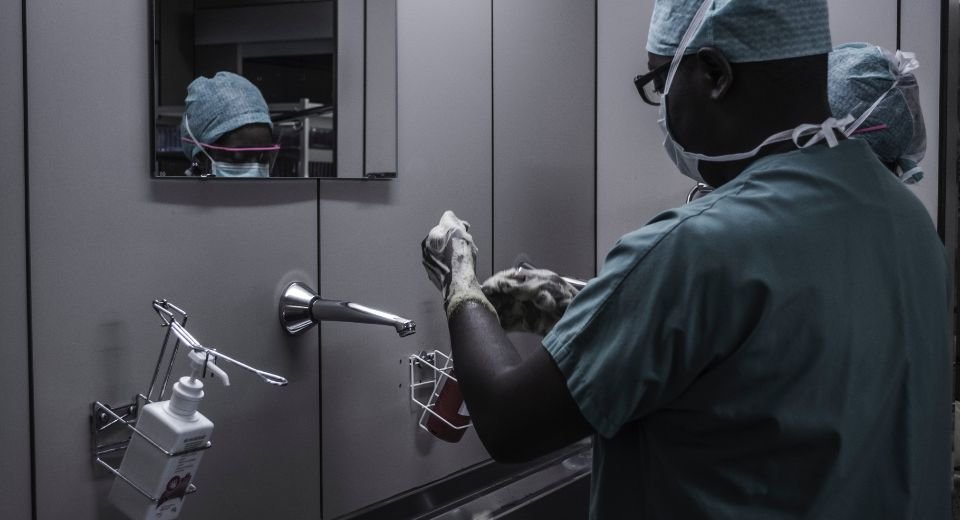
A newly published study in Science Translational Medicine demonstrates that many surgical site infections following spinal surgery originate from microbes already present on the patient’s skin.
Surgical site infections pose a significant challenge in healthcare settings, both economically and clinically. They contribute substantially to the annual costs of hospital-acquired infections and are a leading cause of hospital readmission and mortality post-surgery. A 2013 study found that surgical site infections contribute the most to the annual costs of hospital-acquired infections, totaling over 33 percent of the US$9.8 billion spent annually.
Despite rigorous preventive measures and strict protocols, surgical site infections persist at a concerning rate of about 1 in 30 procedures. Data from the Centers for Disease Control and Prevention show that the problem of surgical site infection is not getting better.
Antibiotic resistance
The rise of antibiotic resistance further complicates infection prevention efforts, with global trends indicating a potential increase in infection rates following surgery. Administering antibiotics during surgery, a cornerstone of infection prevention, becomes less effective as antibiotic resistance spreads.
The research team, comprising multidisciplinary experts from various medical fields, aimed to investigate the root causes of surgical infections despite adherence to preventive protocols. Focusing on spinal surgery due to its prevalence and clinical significance, the study analyzed over 200 patients’ microbiome before surgery and correlated it with subsequent infections.
Remarkably, the study found that 86 percent of bacterial infections post-spinal surgery genetically matched bacteria already present on the patient’s body before surgery. Moreover, nearly 60 percent of these infections showed resistance to preventive antibiotics, indicating pre-existing antibiotic resistance in the patient’s microbiome.
The source of antibiotic resistance was traced back to the patient’s prior exposure to antibiotics, consumer products, or routine community contact, rather than acquisition within the hospital environment.
These findings underscore the importance of infection prevention strategies tailored to individual patients’ microbiomes.
However, further research is needed to assess their impact on patient outcomes. Nevertheless, shifting toward patient-centered, individualized infection prevention measures can pave the way for a more targeted and effective approach to combating hospital-acquired infections in the future.