HQ Team
August 7, 2025: Jazz Pharmaceuticals Plc’s treatment for a rare and aggressive brain tumour that affects children and adults got approval from the US regulator.
The US Food and Drug Administration (FDA) granted an accelerated approval based on an overall response rate in patients with progressive disease following prior therapy, according to a company statement.
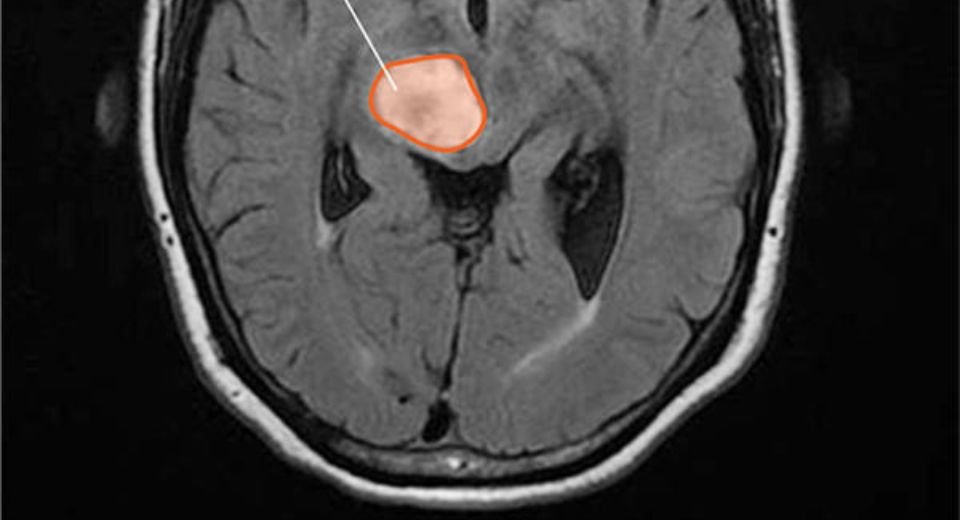
The once-weekly oral drug, dordaviprone, branded as Modeyso, is to treat diffuse midline glioma of the central nervous system in children one year and above and adults. The tumour cells spread out and invade the surrounding healthy brain tissue rather than forming a well-defined mass.
It forms along the midline of the brain or spinal cord, in the brainstem, thalamus, or spinal cord, and originates from glial cells, which are supportive cells in the brain.
Gene mutations
These tumours are associated with specific genetic mutations, which disrupt normal gene regulation and contribute to the aggressive nature of the tumour, which affects 2,000 people in the US every year.
The disease is difficult to treat due to its diffuse nature and sensitive brain locations, with current standard treatment often being radiotherapy to provide symptom relief. The prognosis remains poor, with median survival typically under 15 months after diagnosis.
A continued approval from the FDA depends on “verification and description of clinical benefit” in the end-stage of the trials, according to the statement.
“This is a major turning point in neuro-oncology,” said Patrick Wen, MD, Director, Center for Neuro-Oncology, Dana-Farber Cancer Institute and Professor of Neurology, Harvard Medical School.
“For the first time, we have an FDA-approved therapy for patients with recurrent H3 K27M-mutant diffuse midline glioma. While outcomes remain challenging for many patients, the objective responses observed with dordaviprone, including durable benefit in some patients, represent a meaningful advancement.”
Efficacy analysis
The FDA’s approval was based on an efficacy analysis of 50 patients with recurrent H3 K27M-mutant diffuse midline glioma, selected from five clinical studies based on prespecified eligibility criteria.
The overall response rate, as assessed by blinded independent central review, was 22% and the median duration of response was 10.3 months.
“This approval not only equips clinicians with the first targeted option for this disease but also signals a meaningful shift in what patients and families can expect after diagnosis,” said Joshua E. Allen, PhD, Chief Scientific Officer, Chimerix, a Jazz Pharmaceuticals Company.
The safety of Modeyso was evaluated in 376 adult and pediatric patients with glioma across four clinical studies. The most common adverse reactions in patients were fatigue, headache, vomiting, nausea and musculoskeletal pain.