HQ Team
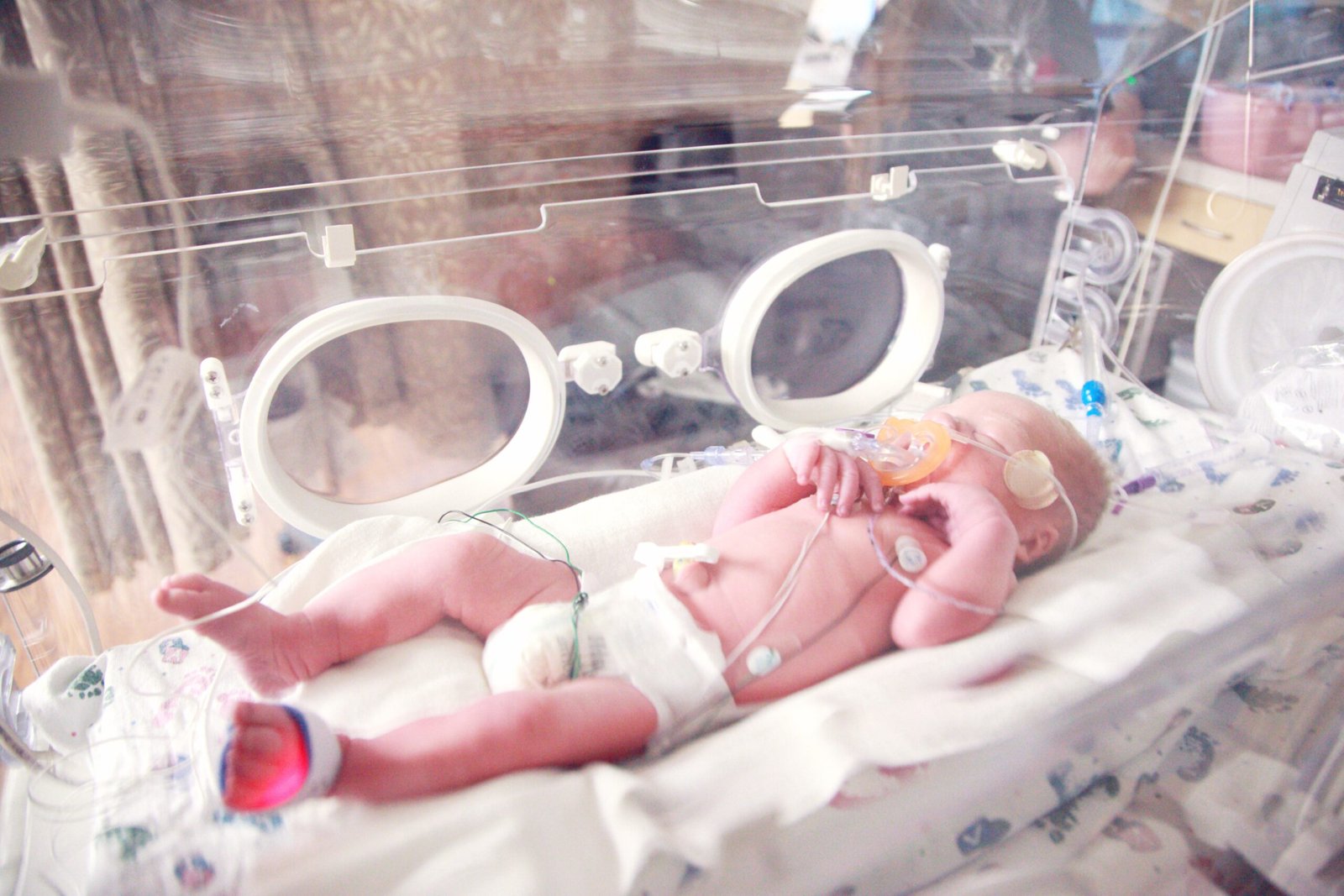
January 16, 2026: An international research team has identified a previously unknown form of diabetes that strikes newborns, revealing critical insights into how genetic mutations can destroy insulin-producing cells within the first six months of life.
The discovery, published in The Journal of Clinical Investigation, pinpoints mutations in the TMEM167A gene as the culprit behind a rare syndrome affecting both metabolism and brain development.
Led by the University of Exeter Medical School and Belgium’s Université Libre de Bruxelles (ULB), the study examined six children who developed diabetes shortly after birth alongside neurological conditions including epilepsy and microcephaly. All six patients shared identical mutations in TMEM167A, establishing what researchers describe as a “monogenic” cause—meaning a single gene error triggers the disease cascade.
“This finding gives us a unique window into the genes essential for insulin production,” said Dr. Elisa de Franco of the University of Exeter. “Identifying these specific DNA changes in babies clarifies how a little-known gene plays a pivotal role in insulin secretion.”
The breakthrough carries weight beyond this rare syndrome. Diabetes now affects 588.7 million adults globally, according to the International Diabetes Federation’s 2025 Atlas, a figure projected to surge 44.81% by 2050. While neonatal diabetes represents a tiny fraction of cases, affecting approximately 1 in 100,000 to 500,000 births, its genetic mechanisms illuminate pathways relevant to all forms of the disease.
Laboratory breakthrough with stem cells
The research team employed cutting-edge stem cell technology to unravel TMEM167A’s function. Professor Miriam Cnop’s ULB laboratory transformed stem cells into pancreatic beta cells—the body’s insulin factories—then used CRISPR gene-editing to replicate the patients’ mutations.
“When TMEM167A is damaged, these cells lose their ability to function and eventually die from internal stress,” Professor Cnop explained. “This stem cell model allows us to study disease mechanisms and test treatments in ways previously impossible.”
The experiments revealed the gene’s surprising specificity: it proves critical for beta cells and neurons but largely irrelevant to most other cell types. This selectivity explains why the disease targets both insulin production and brain development while sparing other organs.
This research arrives as stem cell therapies for diabetes accelerate. From 2000 to 2024, 143 clinical trials explored cell-based treatments, with 83.2% currently in early phases, according to a recent NIH analysis. Vertex Pharmaceuticals’ VX-880 therapy, using stem cell-derived islet cells, recently achieved insulin independence in 83% of Phase 1/2 trial participants, a milestone published in the New England Journal of Medicine.
From rare disease to broad implications
The TMEM167A discovery joins a growing toolkit of genetic insights transforming diabetes care. CRISPR-based therapies recently enabled the first implantation of gene-edited pancreatic cells in a type 1 diabetes patient, who produced insulin for months without immunosuppressive drugs. The study is detailed in WIRED’s September 2025 coverage.
For the 9.5 million people worldwide living with type 1 diabetes—including 1.85 million under age 20—such advances offer tangible hope. Neonatal diabetes itself, when correctly diagnosed, often responds to targeted therapies rather than lifelong insulin injections, making genetic screening potentially life-changing.
“Understanding these rare forms helps us decode the fundamental biology of insulin secretion,” Dr. de Franco noted. “What we learn from infants may inform treatments for the hundreds of millions living with more common diabetes types.”
Research funding
The study received support from Diabetes UK, the European Foundation for the Study of Diabetes, and the Novo Nordisk Foundation, among others.
Researchers emphasize that while TMEM167A mutations cause a rare syndrome, the stem cell model developed here provides a template for investigating other genetic diabetes forms. With diabetes causing over 2 million deaths annually worldwide, according to WHO 2022 data, each genetic clue brings science closer to precision treatments.
“This is an extraordinary model for studying disease mechanisms and testing treatments,” Professor Cnop affirmed. “The ability to generate patient-specific cells revolutionizes how we approach not just rare diabetes, but all forms of this global epidemic.”
Families affected by neonatal diabetes can access genetic testing through specialized centers, with early diagnosis potentially guiding more effective, personalized interventions that could spare children from lifelong insulin dependence and neurological complications.