HQ Team
February 10, 2025: The U.S. Food and Drug Administration (FDA) has approved Emblaveo, a new antibiotic combination of aztreonam and avibactam, for treating adults with complicated intra-abdominal infections (cIAIs) caused by multi-drug resistant bacteria. Developed by AbbVie, the therapy addresses infections where existing treatments are ineffective or unavailable.
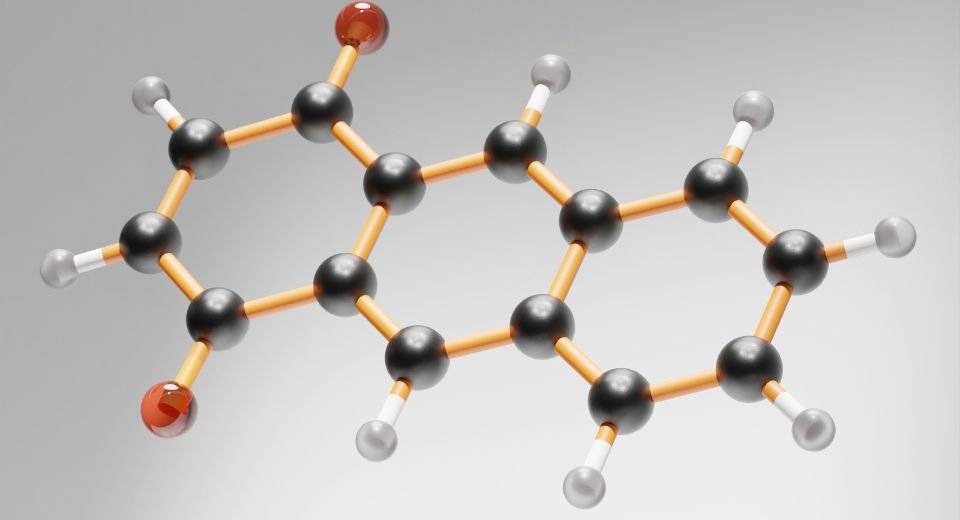
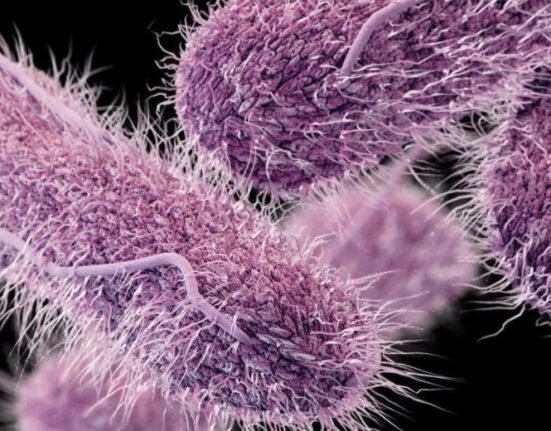
Complicated intra-abdominal infections, such as perforated appendicitis or abscesses, are life-threatening and increasingly linked to antibiotic-resistant pathogens. The World Health Organization (WHO) classifies antimicrobial resistance as a top global health threat, with limited new treatments in development. Emblaveo combines aztreonam, an antibiotic, with avibactam, a beta-lactamase inhibitor that blocks enzymes bacteria use to resist antibiotics. This pairing aims to restore aztreonam’s effectiveness against resistant strains like Enterobacterales, including metallo-beta-lactamase producers.
Clinical trial results
The FDA’s decision followed a Phase 3 trial demonstrating Emblaveo’s efficacy and safety. Patients treated with the drug showed comparable clinical response rates to existing therapies, with a safety profile consistent with aztreonam alone. Common side effects included nausea, headache, and injection-site reactions.
“The continued evolution of antimicrobial resistance among Gram-negative bacteria has left some patients with little to no treatment options, resulting in extended hospital stays, additional morbidity and death,” James A. McKinnell, MD, infectious disease specialist, Milefchik-Rand Medical Group, Torrance Memorial Medical Center, California, said in a statement. “The approval of EMBLAVEO provides physicians a much-needed therapeutic option to help address some of the most difficult antimicrobial-resistant pathogens and provides doctors an opportunity to treat patients with these challenging infections.”
Broader context
cIAIs affect thousands annually in the U.S., with rising resistance to carbapenems and other last-line antibiotics. Emblaveo’s approval aligns with the CDC’s urgent call for innovative antimicrobials.”As bacteria evolve, industry, government, and clinical experts must work together to ensure that the infectious disease community has the tools to advance public health,” Roopal Thakkar, MD, executive vice president, research & development, chief scientific officer, AbbVie, said. AbbVie confirmed the drug will be available in U.S. hospitals by mid-2025, though cost and insurance coverage details remain undisclosed.
While Emblaveo marks progress, experts stress the need for judicious use to prevent further resistance. AbbVie plans additional studies to explore its application in other resistant infections.
This approval underscores the pharmaceutical industry’s role in addressing public health crises, offering hope amid the escalating battle against superbugs.