HQ Team
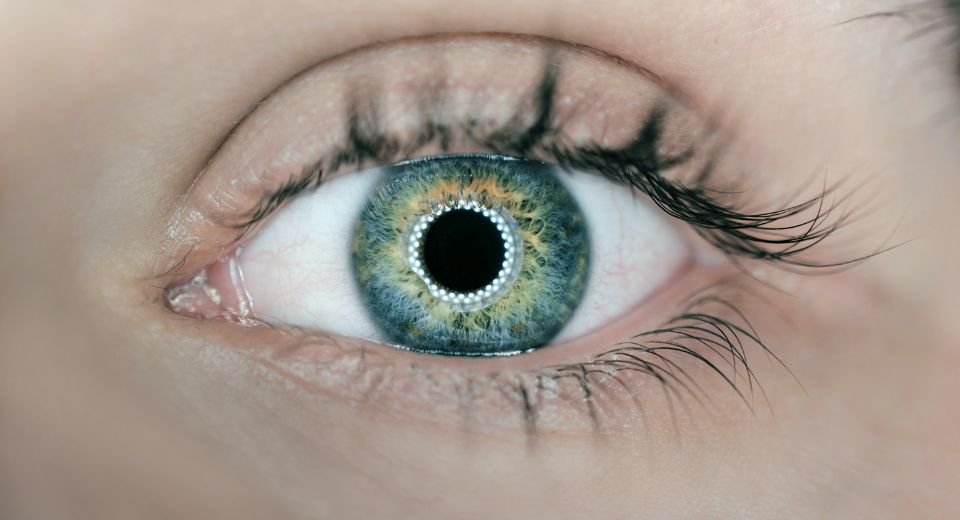
September 12, 2023: The USFDA, in a bid to protect consumers, has issued warning letters to eight companies for marketing illegal and harmful ophthalmic products.
The products are meant to treat eye conditions such as conjunctivitis, cataracts, and glaucoma, among others.
In some cases, the letters cited quality issues related to product sterility.
Boiron Inc., CVS Health, DR Vitamin Solutions, Natural Ophthalmics Inc., OcluMed LLC., TRP Company Inc., Similasan AG/Similasan USA and Walgreen Boots Alliance Inc., were intimated, according to an FDA statement.
Legal action
The FDA has asked the companies to respond within 15 days of receipt of the letters, stating how they will correct the violations.
Failure to correct the violations promptly may result in the FDA pursuing legal action, including product seizure or a court order requiring a company to stop manufacturing and distributing an unapproved product.
The drug regulator has also placed some of these companies on import alert to help stop their products from entering the U.S. and reaching consumers.
The FDA was particularly concerned that these illegally marketed, “unapproved ophthalmic drug products pose a heightened risk of harm to users because drugs applied to the eyes bypass some of the body’s natural defences.”
Some of these eye products were labelled to contain silver, which may be characterised as silver sulfate, silver sulphate or argentum.
Long-term use of drugs containing silver can cause some areas of the skin and other body tissues, including the eye, to permanently turn grey or blue-grey, which is called “argyria.”
Additional actions
Unapproved drugs that claim to cure, treat or prevent serious conditions may cause consumers to delay or stop medical treatments that have been found safe and effective through the FDA review process.
“We will continue to investigate potentially harmful eye products and work to ensure violative products stay off store shelves so that consumers can continue taking the medicines they need without concern,” Jill Furman, director of the Office of Compliance for the FDA’s Center for Drug Evaluation and Research.
The FDA’s investigation of eye products is ongoing, and the agency may take additional regulatory or enforcement actions as warranted, according to the statement.