A study from the Salk Institute has found HIV’s ability to hijack human cells, uncovering a shape-shifting viral protein that could transform treatments for the persistent global health threat.
Published in Nature Communications, the research provides a new structural blueprint of the HIV-1 integrase protein, showing it adopts two dramatically different forms to perform dual functions in the viral life cycle. This discovery opens promising avenues for designing a new class of antiviral drugs that could overcome the persistent challenge of drug resistance .
The Dual Life of a Viral Machine
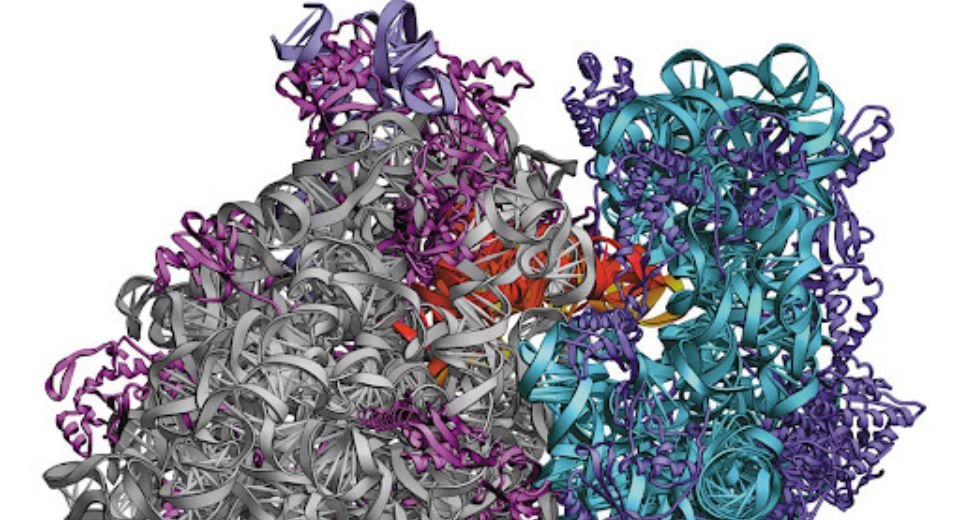
For decades, scientists have known that integrase plays one essential role: it helps insert HIV’s genetic material into the host’s genome, establishing a permanent infection. This process happens through a massive 16-part protein complex called an “intasome” that tightly encircles viral DNA.
The Salk team, using the revolutionary power of cryo-electron microscopy (cryo-EM), discovered that integrase has a second, completely separate function. Later in the viral replication process, it disassembles from the large DNA-handling complex and transforms into a minimalist, four-part complex that binds to viral RNA inside the HIV capsid .
“This marks the first time researchers have visualized integrase’s RNA-bound state,” the study highlights, providing a high-resolution blueprint for a form that was previously a mystery to science .
When a virus infects a cell, it inserts its genetic material (DNA or RNA) into the host genome and hijacks the cell’s machinery to make more of the virus. Most viruses that use RNA are like outsiders who have to bring their own tools because our cells are only designed to read and copy DNA. But HIV is a special kind of virus called a retrovirus, and it has a sneaky trick. Once it gets inside a cell, it uses its own RNA to create a DNA copy. This viral DNA then slips into the cell’s own instructions, hiding there. From that point on, the hijacked cell is fooled into thinking the viral blueprint is part of itself, and it becomes a factory that churns out new copies of the virus, which then go on to infect even more cells.
New Front in the Battle Against Drug Resistance
The discovery that integrase is a multifunctional protein is a game-changer for drug development. Current integrase inhibitors, such as Dolutegravir, are effective but only target the protein’s DNA integration role. HIV’s rapid mutation rate often allows the virus to develop resistance to these drugs over time .
This new research suggests that by developing drugs that also target integrase’s secondary role in RNA interaction, scientists could attack the virus from a new angle.
“Mapping its interaction with RNA not only enriches our knowledge of viral biology but also informs the rational design of next-generation HIV therapeutics,” said senior author Dr. Dmitry Lyumkis .
The structural insights reveal how subtle shifts in the protein’s structure allow it to pivot between its DNA and RNA binding functions. This inherent flexibility means that future pharmaceuticals could be designed to trap the protein in an inactive state, effectively crippling the virus at multiple stages of its life cycle .
Structural Biology
This research underscores how advances in structural biology are directly fueling therapeutic innovation. The cryo-electron microscopy technology used by the team allows scientists to visualize large, flexible protein complexes in near-atomic detail, a feat that was impossible just years ago .
“The high-resolution cryo-EM maps generated by the researchers provide unparalleled detail into integrase’s architecture, setting a new standard for future investigations of viral proteins,” the study reports.
The findings are the result of a significant collaboration among experts in molecular biology, structural biology, and virology from several institutions, including the University of Colorado School of Medicine and the Dana-Farber Cancer Institute
As the scientific community digests these findings, the next steps will involve confirming the precise mechanisms of integrase’s RNA interactions and exploring how to therapeutically target these processes. This more complete picture of HIV’s complex replication machinery promises to reshape drug development paradigms for years to come, offering new hope in the enduring fight against the global HIV pandemic .