HQ Team
July 1, 2023: Researchers at the University of Cambridge have moved away from cells of zebrafish and mice to create a “human embryo-like model” from human cells to predict early-stage pregnancy failures.
In natural humans, the second week of development is an important time when the embryo implants into the uterus and is the time when many pregnancies are lost.
The new model enables scientists to peek into the mysterious ‘black box’ period of human development – usually following implantation of the embryo in the uterus – to observe processes never directly observed before.
It allows experimental modeling of embryonic development during the second week of pregnancy.
Researchers can gain knowledge of the developmental origins of organs and specialized cells, such as sperm and eggs, and facilitate understanding of early loss during pregnancy.
Birth defects
Understanding the early developmental processes holds the potential to reveal some of the causes of human birth defects and diseases, and to develop tests for these in pregnant women.
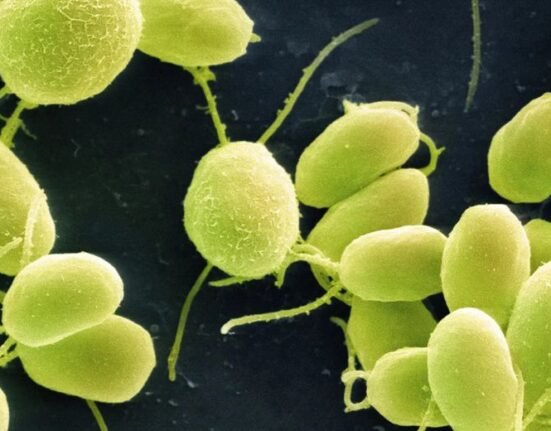
The model is an organized three-dimensional structure derived from pluripotent stem cells or cells capable of producing different cell types that replicate some developmental processes that occur in early human embryos.
“Our human embryo-like model, created entirely from human stem cells, gives us access to the developing structure at a stage that is normally hidden from us due to the implantation of the tiny embryo into the mother’s womb,” said Prof Magdalena Zernicka-Goetz at the University of Cambridge’s Department of Physiology, Development, and Neuroscience, who led the work.
The model “allows us to manipulate genes to understand their developmental roles in a model system. This will let us test the function of specific factors, which is difficult to do in the natural embryo.”
Legal restrictions in the UK prevent the culture of natural human embryos in the lab beyond day 14 of development. This time limit was set to correspond to the stage where the embryo can no longer form a twin.
Donated human embryos
Until now, scientists have only been able to study this period of human development using donated human embryos. The new model could reduce the need for donated human embryos in research.
While these models can mimic aspects of the development of human embryos, they cannot and “will not develop to the equivalent of postnatal stage humans,” Prof Zernicka-Goetz said.
In 2021 and in 2022 her team announced in Developmental Cell, Nature, and Cell Stem Cell journals that they had finally created model embryos from mouse stem cells that can develop to form a brain-like structure, a beating heart, and the foundations of all other organs of the body.
The current model derived from human stem cells do not have a brain or beating heart, but they include cells that would typically go on to form the embryo, placenta, and yolk sac, and develop to form the precursors of germ cells — that will form sperm and eggs.
Signals to each other
Many pregnancies fail at the point when these types of cells orchestrate implantation into the uterus and begin to send mechanical and chemical signals to each other, which tell the embryo how to develop properly.
There are clear regulations governing stem cell-based models of human embryos and all researchers doing embryo modeling work must first be approved by ethics committees.
Journals require proof of this ethics review before they accept scientific papers for publication. Zernicka-Goetz’s laboratory holds these approvals.
“It is against the law and FDA regulations to transfer any embryo-like models into a woman for reproductive aims. These are highly manipulated human cells and their attempted reproductive use would be extremely dangerous,” said Dr Insoo Hyun, a member of Harvard Medical School’s Center for Bioethics.
The research was funded by the Wellcome Trust and Open Philanthropy and published in the journal Nature.