HQ Team
June 22, 2025: Madrigal Pharmaceuticals EU Limited’s therapy to treat adults with noncirrhotic metabolic dysfunction-associated steatohepatitis (MASH) got a conditional marketing authorisation in the European Union.
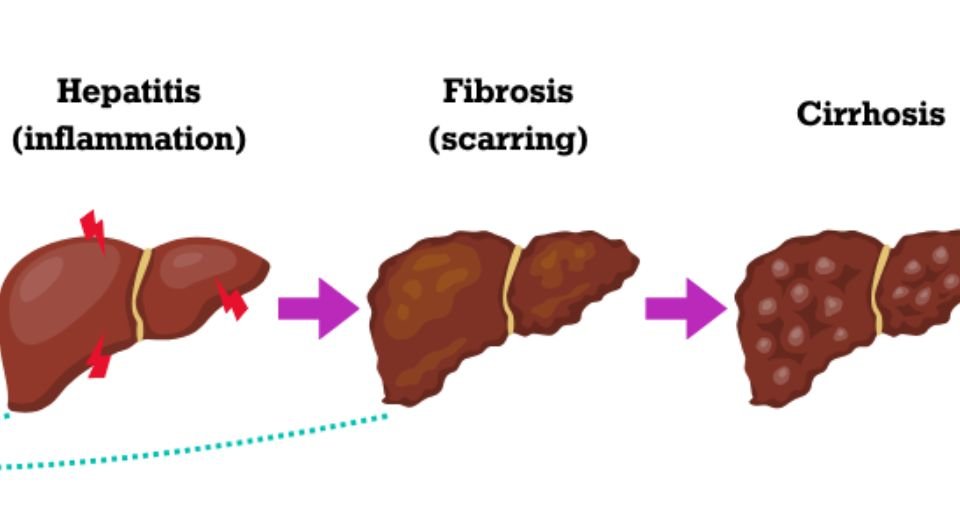
The substance responsible for the activity of Rezdiffra is resmetirom, which stimulates the thyroid hormone receptor beta (THR‑β) in the liver, reducing lipotoxic liver fat, inflammation and liver fibrosis — tissue scarring and thickening.
TRβ is predominantly found in the liver, kidneys, pituitary gland, and brain, where it regulates thyroid hormone effects on metabolism, cholesterol, and lipid homeostasis.
The Committee for Medicinal Products for Human Use (CHMP) “adopted a positive opinion, recommending the granting of a conditional marketing authorisation for rezdiffra,” according to a statement.
The benefits of the therapy are the resolution of MASH as well as improvement in fibrosis compared with placebo, as observed in a clinical trial in patients with MASH and liver fibrosis.
Diet, exercise
The most common side effects with Rezdiffra include diarrhoea, nausea, and itching. It is to be administered in conjunction with diet and exercise.
Rezdiffra will be available in formulations of 60 mg, 80 mg and 100 mg film-coated tablets.
A conditional marketing authorisation is granted to a medicinal product that fulfils an unmet medical need when the benefit to public health of immediate availability outweighs the risk inherent in the fact that additional data are still required.
The marketing authorisation holder is expected to provide comprehensive clinical data at a later stage.
MASH is a serious disease where fat deposits accumulate in the liver, causing inflammation. MASH is often associated with several diseases of the heart and blood vessels and metabolic diseases, and, if left untreated, it can lead to cirrhosis — severe and permanent scarring of the liver and cancer.
Pivotal clinical study
Presently, there is no authorised treatment for MASH in the EU.
The opinion of the European Medicines Agency’s human medicines committee (CHMP) was based on data from a pivotal clinical study. The applicant submitted the results of an interim analysis based on a subset of participants after one year of treatment.
The study included a total of 917 patients with liver fibrosis stage F2 (moderate) and F3 (advanced) at baseline.
Of the total 306 participants received 80 mg of resmeritom, 308 was administered 100 mg of the same drug and 303 received a dummy drug or placebo.
Results after 12 months showed that 30% of patients in the 100 mg resmeritom and 26% of those in the 80 mg group achieved MASH resolution with no worsening of fibrosis, compared to 10% in the placebo group.
Twenty-nine per cent of patients who received 100 mg of resmeritom and 27% of those who received 80 mg of resmeritom experienced an improvement in liver scarring (fibrosis) and no worsening of MASH, compared to 17% of those who received the placebo or a dummy drug.
Further data on efficacy
The CHMP considered that the results of the main trial demonstrated the efficacy of Rezdiffra.
The conclusion on the beneficial effects was supported by the submission of two studies conducted in a slightly different population, which “partly show similar features” to the primary target population.
Since the pivotal trial, and one of the two supportive trials, are ongoing, the CHMP has required the applicant to complete these two studies as they are necessary to provide further data regarding the efficacy of Rezdiffra.
The CHMP’s opinion will now be sent to the European Commission for the adoption of a decision on an EU-wide marketing authorisation.
Once a marketing authorisation has been granted, decisions about price and reimbursement will take place at the level of each Member State.
Resmetirom, marketed as rezdiffra, was approved by the US FDA on March 14, 2024.