HQ Team
February 6, 2025: The U.S. Food and Drug Administration (FDA) issued a critical Class I recall—the most severe category—for Medtronic’s Becker and Exacta’s External Drainage and Monitoring Systems following reports of device cracks and leaks.
The leaks can lead to life-threatening infections, cerebrospinal fluid (CSF) leaks, and patient deaths. At least 15 injuries have been tied to the devices, which are used to drain excess fluid from the brain in critical care settings.
Recall advisory
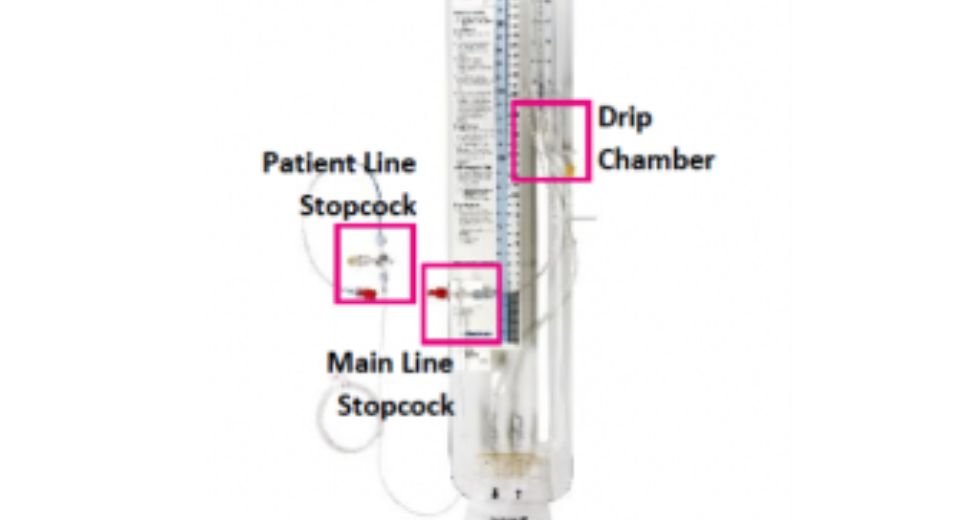
Medtronic has not removed the devices from the market but is urging healthcare providers to immediately inspect their inventory. The company advises clinicians to check all stopcocks and tubing connections for visible cracks, leaks, or looseness before use. Devices showing defects should be quarantined and returned to Medtronic.
Class I recalls signal “a reasonable probability that product use will cause serious adverse health consequences or death,” according to the FDA. The affected Medtronic systems, designed to monitor and drain CSF in patients with conditions like hydrocephalus or traumatic brain injuries, are critical for preventing dangerous pressure buildup in the skull. However, cracks in stopcocks (valves controlling fluid flow) or loose connections can lead to CSF leaks, exposing patients to bacterial infections such as meningitis. In severe cases, such complications may prove fatal.
A persistent challenge in medical devices
This recall underscores broader challenges in the medical device industry, where even minor design flaws can have catastrophic outcomes. Medtronic, a global leader in medical technology, has faced prior recalls, including 2022’s urgent correction of insulin pump controllers. The latest action highlights the vulnerability of external drainage systems, which require precision engineering to maintain sterility and function under constant use.
The FDA’s announcement did not specify whether the reported injuries included fatalities, but the agency emphasized that providers must “act swiftly” to mitigate risks. Patients reliant on these systems are often already in critical condition, making device reliability non-negotiable.
Hospitals and clinics using Becker or Exacta systems should:
- Inspect devices immediately: Focus on stopcock valves and connection points.
- Report defects: Contact Medtronic for returns.
- Monitor patients: Watch for signs of infection, sudden headaches, or changes in CSF output.
Patients or families are advised to consult their healthcare teams if concerned about device safety. While Medtronic has not recommended discontinuing use outright, heightened vigilance is critical.
Broader implications for medical safety
This recall reinforces the importance of post-market surveillance, as even rigorously tested devices can develop issues over time. The FDA encourages providers to report adverse events through its MedWatch portal to improve real-time data collection.
For Medtronic, the recall may prompt renewed scrutiny of its quality control processes. The company stated it is “working closely with the FDA” to address the issue but has yet to announce design changes or a timeline for resolving the defects.
As the FDA’s strictest recall category, this Class I warning serves as an urgent reminder of the delicate balance between innovation and patient safety in medical technology.