HQ Team
February 24, 2025: American-Irish Medtronic Plc’s deep brain stimulation device for patients with Parkinson’s disease (PD) has been approved by the US Food and Drug Administration, according to a company statement.
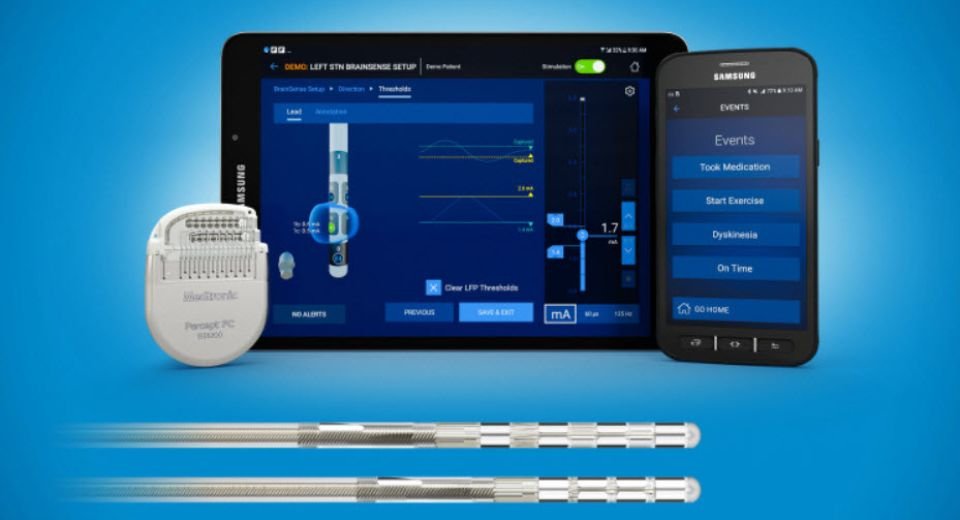
BrainSense’s stimulation is similar to what a cardiac pacemaker is to the heart.
The device uses a surgically implanted neurostimulator through a minimally invasive procedure to transmit electrical signals to specific parts of the brain affected by debilitating neurological disorders such as Parkinson’s.
For Parksinson’s, the Minneapolis, Minnesota-headquartered company has added a feature that personalises therapy based on a patient’s brain activity in real-time – both in clinical settings and daily life.
The BrainSense Adaptive deep brain stimulation (DBS) provides enhanced therapy personalisation for symptom control that automatically adjusts, instead of patients doing it manually.
Neurostimulator
The BrainSense Adaptive DBS is available to Medtronic DBS patients with Parkinson’s who have been implanted with a Percept neurostimulator, as well as new patients, according to the statement.
Parkinson’s disease is a progressive movement disorder of the nervous system. It causes nerve cells (neurons) in parts of the brain to weaken, become damaged, and die, leading to symptoms that include problems with movement, tremors, stiffness, and impaired balance. As symptoms progress, people with the disease may have difficulty walking, talking, or completing other simple tasks.
“Medtronic is the only company in the world to offer an adaptive DBS system that dynamically adjusts therapy in real-time,” said Brett Wall, executive vice president and president of the Medtronic Neuroscience Portfolio.
“This new era in Parkinson’s care represents more than a decade of intentional innovation—ushering in personalised neuromodulation at a scale that responds to a patient’s changing needs, equipping clinicians with unparalleled insights, and setting a new standard for DBS therapy.”
10 million worldwide
More than 10 million people worldwide are living with Parkinson’s, according to Parkinson’s Foundation. The incidence of Parkinson’s disease increases with age, but an estimated four per cent of people with the disease are diagnosed before age 50.
Nearly one million people in the US are living with Parkinson’s. This number is expected to rise to 1.2 million by 2030. Parkinson’s is the second-most common neurodegenerative disease after Alzheimer’s disease.
“Adaptive deep brain stimulation will help revolutionise the approach to therapeutic treatment for patients with Parkinson’s disease,” said Helen Bronte-Stewart MD MSE, FAAN, FANA, John E. Cahill Family Professor in the Department of Neurology and Neurological Sciences and Director of the Human Motor Control and Neuromodulation Lab at Stanford University School of Medicine.
“The transformative personalized care we can achieve through automatic adjustment greatly benefits patients receiving therapy that adapts to their evolving needs.”
Electrode Identifier
The US FDA approval also includes the Medtronic BrainSense Electrode Identifier device, which helps reduce patient time spent in the clinic to program their DBS settings.
By using the identifiers, clinicians can conduct accurate and precise initial programming 85% faster compared to traditional electrode selection, according to the company statement. Medtronic’s legal headquarters are in Ireland due to its acquisition of Irish-based Covidien in 2015.