HQ Team
November 4, 2023: Scientists have discovered a possible treatment for antibiotic-resistant super gonorrhea. In a large experiment involving nearly a 1000 people, the experimental drug zoliflodacin demonstrated strong efficacy in fighting the stubborn bacterial infection.
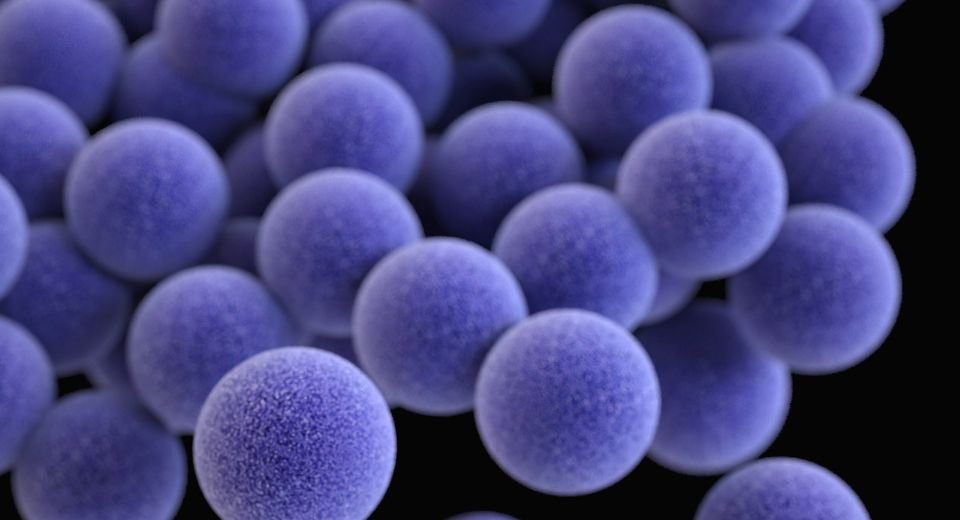
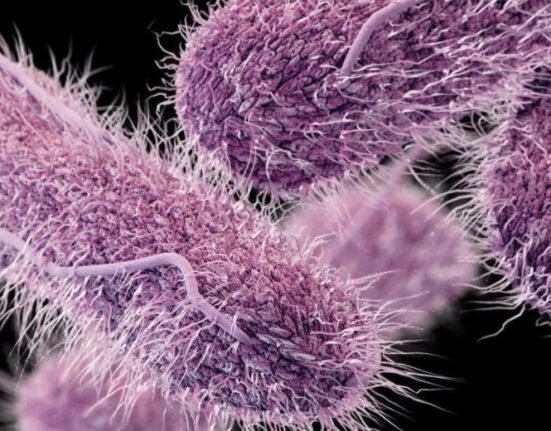
Gonorrhea, caused by the Neisseria gonorrhoeae bacteria, is a sexually transmitted infection with symptoms, including genital discharge, painful urination, and complications if left untreated. It’s also linked to infertility, increased risk of other STIs, and neonatal blindness. The once-easily treatable disease has evolved to resist common antibiotics, posing a growing public health threat.
WHO estimates that in 2020, there were 82.4 million [47.7 million-130.4 million] new cases infected among adolescents and adults aged 15–49 years worldwide.
Evolution of antibiotic resistance in gonorrhea
Gonorrhea has become increasingly resistant to antibiotics, raising concerns about the emergence and spread of superbugs. The Covid-19 pandemic added to the problem, with people avoiding hospitals and self medicating, which led to antibiotic misuse. The global struggle against antibiotic resistance has driven this quest for a super-fighting antibiotic.
Zoliflodacin’s unique approach
Zoliflodacin, developed by the Global Antibiotic Research & Development Partnership (GARDP) in collaboration with Entasis Therapeutics, has shown promise in this regard. It employs a unique mechanism to eliminate gonorrhea bacteria and effectively targets drug-resistant strains.
Successful Phase III clinical trial
A Phase III clinical trial involving over 900 individuals in multiple countries demonstrated zoliflodacin’s efficacy in treating uncomplicated gonorrhea. Results indicated that it cleared infections as effectively as traditional treatments. The drug was well-tolerated, with no reported serious adverse events or deaths.
Experts say zoliflodacin’s potential cross-resistance with other antibiotics can simplify gonorrhea treatment.
Drug approval
While the findings await further trials and regulatory overseeing, zoliflodacin is well-positioned for fast-track drug approval. If successful, it would mark the first new gonorrhea drug developed in decades and could also be used to treat other STIs like chlamydia.
GARDP’s commitment to commercializing zoliflodacin in most parts of the world will ensure accessibility. To prevent resistance, it’s crucial to use the drug judiciously.