HQ Team
May 9, 2025: A steroid-free topical foam has demonstrated strong efficacy in treating psoriasis on both the scalp and body, according to a Phase 3 clinical trial study. The research found that daily use of roflumilast foam 0.3% (marketed as Zoryve) led to significant improvements in itch relief and plaque clearance, offering hope for patients who struggle with managing psoriasis in these challenging areas.
The study involved 432 patients (aged 12+) with moderate-to-severe scalp and body psoriasis, randomly assigned to use either roflumilast foam or a placebo foam for eight weeks. Results showed:
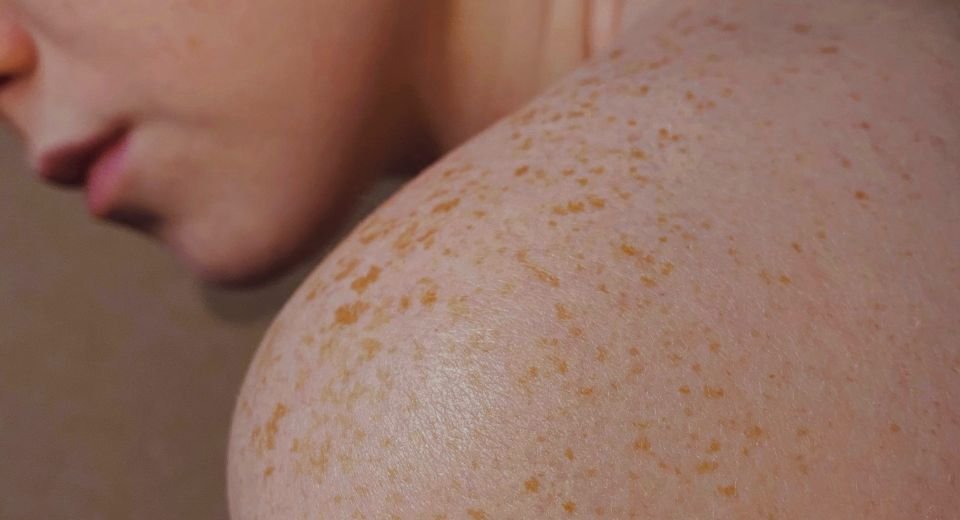
Scalp Psoriasis (S-IGA Success): 66.4% of roflumilast users achieved “clear” or “almost clear” skin, compared to 27.8% in the placebo group.
Body Psoriasis (B-IGA Success): 45.5% saw significant improvement vs. 20.1% with placebo.
There was rapid itch relief in the scalp and body within 24 hours of the first application.
The side effects included mild headache (4.6%) and nausea (2.1%).
“Roflumilast foam’s efficacy aligns with earlier studies on its cream formulation, offering a much-needed single treatment for dual-area psoriasis,” the researchers noted.
Treating psoriasis, a chronic inflammatory skin condition characterized by thick, scaly, red patches that can be itchy and painful. Psoriasis affects over 125 million people globally (WHO, 2024), with up to 80% experiencing scalp involvement (National Psoriasis Foundation). Current treatments often require separate prescriptions for scalp and body due to formulation limitations (e.g., creams vs. solutions). Roflumilast foam, a PDE4 inhibitor, reduces inflammation without steroids, making it suitable for long-term use.
Dr. Mark Lebwohl (Mount Sinai, NYC, uninvolved in the study) commented: “A steroid-free option that works on both areas is a significant advance. Patients often juggle multiple treatments, which affects adherence.”
Roflumilast targets phosphodiesterase-4 (PDE4), an enzyme linked to inflammation. By dampening immune overactivity and blocking itch signals, it addresses:
Plaque buildup (rapid skin cell turnover). And itch-sensory nerves (providing quick relief). The foam formulation penetrates hair-covered scalp areas effectively, unlike creams or ointments.
The present trial was of 8-week durations, long-term effects remain under study. Roflumilast foam is FDA-approved for seborrheic dermatitis but not yet for psoriasis. Patients may need to consult dermatologists for prescriptions. Industry analysts predict FDA approval for psoriasis by 2025, pending further trials.
The study was published in the journal JAMA Dermatology.