HQ Team
January 18, 2024: The FDA has approved US-based DermaSensor Inc.’s handheld wireless skin cancer detection device that is powered by artificial intelligence technology.
Primary care physicians’ current options for evaluating suspicious moles have been the naked eye or magnified visual examination of lesions, both of which are dependent on clinical training and subjective judgment.
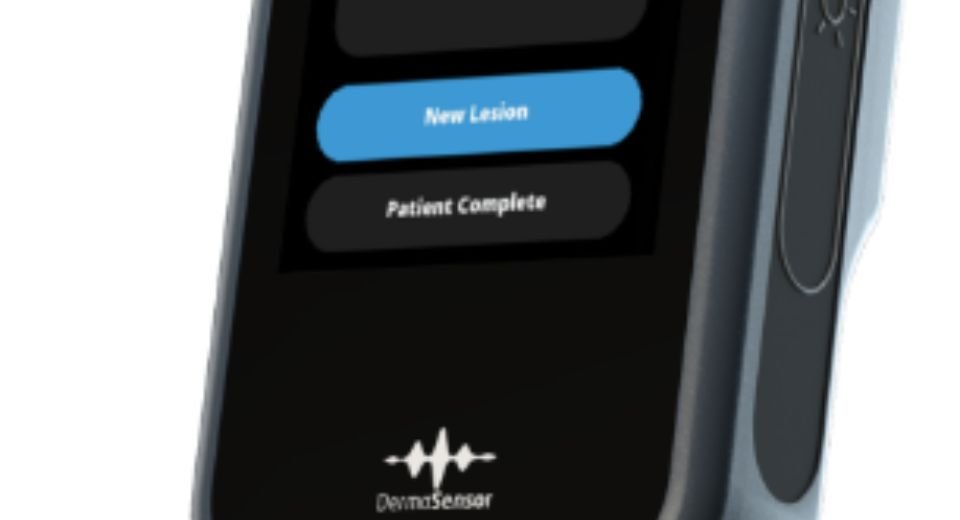
“DermaSensor’s AI-powered spectroscopy technology allows them to non-invasively evaluate cellular and sub-cellular characteristics of a lesion in question for skin cancer,” according to a company statement. “The wireless, handheld device provides an immediate, objective result using an FDA-cleared algorithm.”
The device can be used to test all forms of skin cancer.
224 skin cancers
The US drug regulator’s green light was based on a study that showed the device had a sensitivity of 96% across all 224 skin cancers. A negative result had a 97% chance of being benign for all skin cancers, according to the statement.
The device called DermaSensor has a tip that reflects and records quick bursts of light off the lesion’s cellular and sub-cellular content.
The light is analysed by the built-in computer to provide information to help physicians assess skin lesions, including melanomas, squamous cell carcinomas, and basal cell carcinomas, to aid in a referral decision.
By examining the difference in light scattering, DermaSensor determines if the skin lesion is “Investigate Further” or “Monitor,” an immediate output.
DermaSensor uses elastic scattering spectroscopy, a process that evaluates how photons scatter when reflected off of different cellular structures.
Early detection
Malignant lesions have been reported to have different cellular and sub-cellular structures than benign lesions, scattering light differently.
One out of five Americans will have had some type of skin cancer by the age of 70, and the annual cost of treating skin cancers in the US is estimated at $8.1 billion, with an estimated 5.5 million new cases each year.
About 99% of skin cancers, including the most deadly form, melanoma, are curable if detected early.
Access to dermatology is challenging, especially in rural areas, which makes empowering primary care to identify those cases warranting a referral even more vital.
‘Unmet need’
“We are entering the golden age of predictive and generative artificial intelligence in healthcare, and these capabilities are being paired with novel types of technology, like spectroscopy and genetic sequencing, to optimise disease detection and care,” said Cody Simmons, co-founder and Chief Executive Officer of DermaSensor.
“Equipping primary care physicians, the most abundant clinicians in the country, to better evaluate the most common cancer in the country has been a major, long-standing unmet need in medicine.”
The privately held company has conducted 13 clinical studies in the last decade, six of which provided the principal support for FDA clearance. DermaSensor is currently commercially available in Europe and Australia.