HQ Team
February 4, 2023: A probe by the US-based Centres for Disease Control into the deaths of 70 children in Gambia “strongly suggests” cough syrups involving contaminated diethylene glycol and ethyl glycol, by an Indian company, were involved.
“This investigation strongly suggests that medications contaminated with diethylene glycol and ethylene glycol imported into Gambia led to,” an acute kidney injury outbreak among children, according to a CDC report.
Laboratory analysis of 23 medication samples by Gambia’s Ministry of Health, and WHO, confirmed that four products from Maiden Pharmaceuticals Limited, Haryana, India, contained diethylene glycol and ethylene glycol.
Acute kidney injury outbreaks associated with diethylene-contaminated pharmaceutical products by different manufacturers have been documented in Panama, Nigeria, India, and Haiti.
Patients with diethylene glycol poisoning can experience a range of signs and symptoms, including altered mental status, headache, abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, and acute kidney injury, which may lead to death.
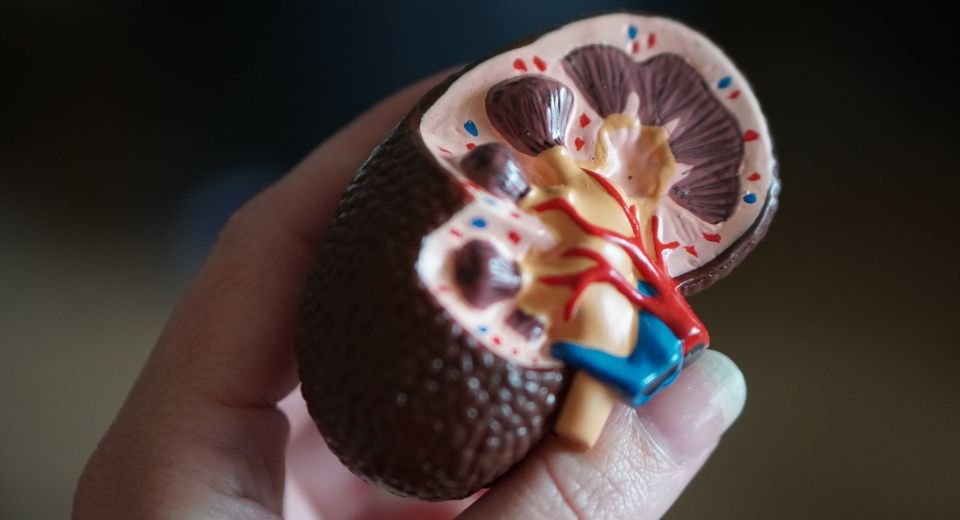
Acute kidney injury
The most consistent manifestation is acute kidney injury, characterized by low urine output or anuria, progressing to renal failure over one to three days.
On July 26, 2022, a pediatric nephrologist on July 26 last year had alerted The Gambia’s Ministry of Health to a cluster of cases of acute kidney injury among young children at the country’s only teaching hospital. On August 23, 2022, the ministry requested assistance from CDC.
In October, Gambia’s Ministry of Health suspended the importation of all medications from Maiden Pharmaceuticals and requested that healthcare providers stop prescribing, dispensing, and using medicines produced by the Indian company because of their possible contamination.
The next day the World Health Organisation issued a worldwide medical product alert for four syrup-based medications from Maiden Pharmaceuticals.
Madien has denied the charges, and the Indian government said the syrups showed no contamination when tested in a state-run laboratory. The company has stopped production at its factory.
Review of records
The CDC investigators reviewed medical records and interviewed caregivers to characterize patients’ symptoms and identify exposures.
Initial reports from the physicians at the teaching hospital indicated that caregivers had administered paracetamol and promethazine to their children before they developed acute kidney injuries.
Based on records from Gambia’s Medicines Control Agency, all medications tested positive for the contaminants were imported into Gambia on June 21, 2022, shortly before the first kidney cases.
Domestic manufacturers
Among reports of acute kidney injury associated with diethyelene-contaminated medical products, this is the first in which the contaminated medications were imported into a country rather than being domestically manufactured, according to the report.
“This likely poisoning event highlights the potential public health risks posed by the inadequate quality management of pharmaceutical exports.
“Inadequate regulatory structures make the sale of medications from international markets an especially high-risk activity in low-resource settings.”
Maiden’s products include Promethazine Oral Solution, Kofexmalin Baby Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup.