HQ Team
October 18, 2023: Hyloris Pharmaceuticals SA, Liège, Belgium-based biopharma company, announced the US Food and Drug Administration has approved its post-operative pain treatment drug.
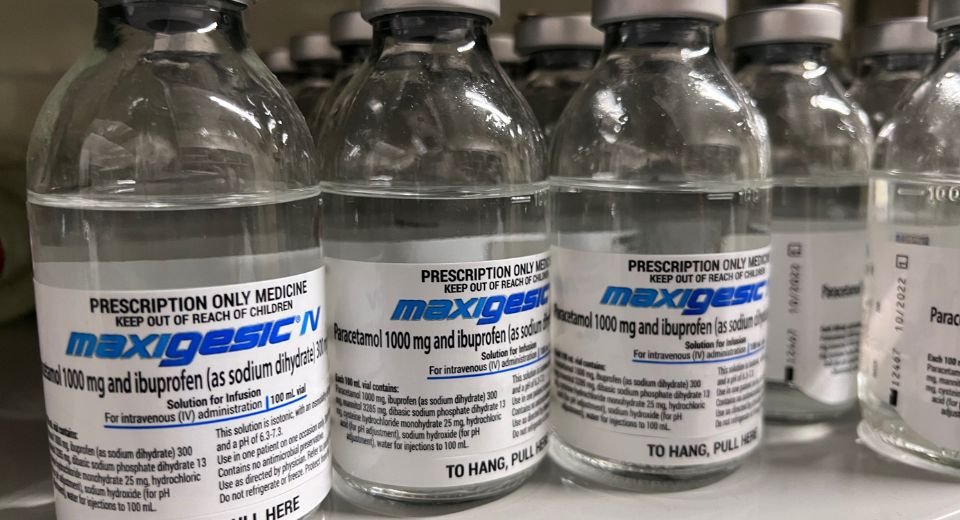
The non-opioid injection, Maxigesic IV to be branded as Combogesic IV in the US market, will be available in early 2024, according to a company statement.
The FDA approval came after Maxigesic IV proved to be “well-tolerated and offered faster onset of action and higher pain relief compared to Paracetamol IV (Acetaminophen IV) and Ibuprofen IV, as well as placebo.”
The approval for the New Drug Application was based on positive data from a Phase 3 program in which the analgesic effect of the drug was supported by a range of secondary endpoints, including reduced opioid usage rates.
Combination
The Maxigesic injection is a combination of paracetamol and ibuprofen solution to be used for post-operative infusion.
“Maxigesic IV demonstrates the potential of our strategy of bringing product candidates to market within our strict criteria: a development of 7 years or less, and R&D costs averaging less than 7 million euros,” said Stijn Van Rompay, Chief Executive Officer of Hyloris.
The drug will be sold in the US through a license and distribution agreement between Hyloris’ partners AFT Pharmaceuticals and Hikma Pharmaceuticals.
Under the terms of the development collaboration agreement between Hyloris and AFT, Hyloris is eligible to receive a share on any product-related revenues, such as license fees, royalties and milestone payments, received by AFT.
$3.6 million payments
Hyloris will be entitled to a milestone payment of $2.1 million and an additional payment of $1.5 million relating to existing trade receivables is expected.
The development of chronic opioid use after surgery is one of the most common postoperative complications with particularly devastating consequences. In the US, the prevalence of new chronic opioid use after surgical procedures was estimated at close to 6%.
In the past two decades, prescription opioid usage in the U.S. contributed to over 600,000 deaths related to opioid overdoses, with the annual death toll rising tenfold between 1999 and 2021 (80,000 deaths in 2021).
Nearly 17,000 deaths involved prescription opioids in 2021.
Patients requiring medical attention related to opioid abuse account for around $11 billion of added costs to the US healthcare system annually, or 1% of all hospital costs.
Post-operative pain is a normal response to surgical intervention and is a cause of delayed recovery and discharge after surgery.
The global post-operative pain therapeutics market reached a value of $12.6 billion in 2023 and is anticipated to grow at a CAGR of 4.7% to reach a value of $19 billion by 2032, with the US as the largest market.