HQ Team
December 12, 2022: Researchers, who conducted two clinical trials, have developed a therapy that makes the immune system destroy bone marrow cancer cells.
The therapy worked on 73% of the patients studied by researchers from the Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai.
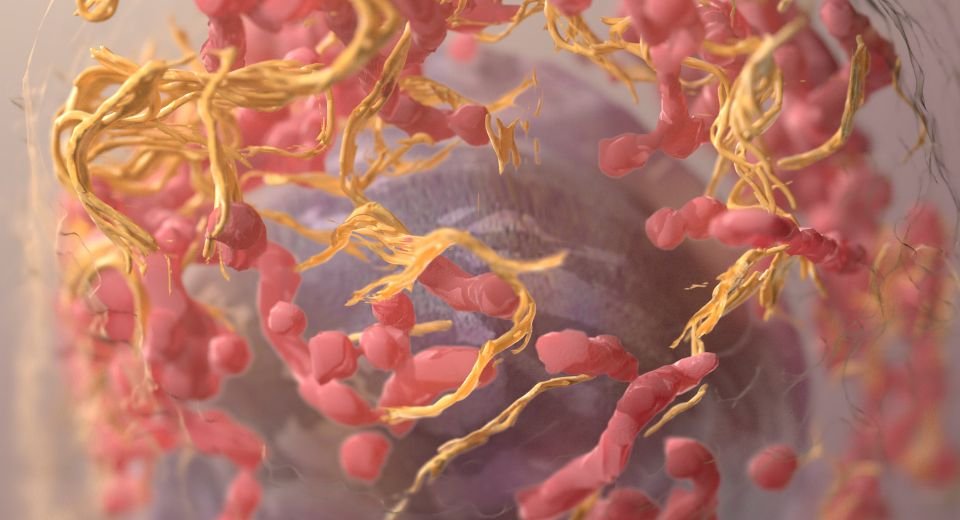
They found the therapy, known as a bispecific antibody, binds both T cells and multiple myeloma cells and commands the T cells to decimate the cancer cells.
T cells are part of the immune system and develop from stem cells in the bone marrow. They help protect the body from infection and may help fight cancer. The researchers termed this strategy “bringing your army right to the enemy.”
The success of the off-the-shelf immunotherapy, called talquetamab, was even seen in patients whose cancer was resistant to all approved multiple myeloma therapies, according to EurekAlert.
It uses a different target than other approved therapies, a receptor expressed on the surface of cancer cells known as GPRC5D.
New hope
Talquetamab was tested in phase 1 and phase 2 trials. The study participants had all been previously treated with at least three different therapies without achieving lasting remission, suggesting talquetamab could offer new hope for patients with hard-to-treat multiple myeloma.
“This means that almost three-quarters of these patients are looking at a new lease on life,” said Ajai Chari, lead author of both studies.
“Talquetamab induced a substantial response among patients with heavily pretreated, relapsed, or multiple refractory myelomas, the second-most-common blood cancer. It is the first bispecific agent targeting the protein GPRC5d in multiple myeloma patients.”
Most patients with myeloma who receive standard therapies continually relapse. Patients who relapse or become resistant to all approved multiple myeloma therapies have a poor prognosis, so additional treatments are needed.
This study, an early-phase trial designed to detect tolerability and find a safe dose, is an essential step in meeting that need, according to EurekAlert.
Focus on doses
The phase 1 clinical trial enrolled 232 patients at several cancer centres worldwide between January 2018 and November 2021. Patients received a variety of doses of the therapy intravenously or injected under their skin.
Future studies will focus on doses only administered under the skin weekly or every other week.
The researchers validated the efficacy and safety findings in phase 1 in the phase 2 trial. The phase 2 trial included 143 patients treated on a weekly dose and 145 at a higher biweekly dose.
Dr Chari said the response rate in these two groups was about 73%.
Researchers continue to collect data on the duration of response in the group receiving 0.8 mg/kg every other week and for patients in both dosing groups who had a complete response or better.
Side effects were relatively frequent but typically mild. About three-quarters of patients experienced cytokine release syndrome, a constellation of symptoms including fever that is common with immunotherapies.