HQ Team
January 23, 2025: China’s YolTech Therapeutics, a biotechnology startup, has started its clinical trial of an investigational therapy for treating transfusion-dependent beta-thalassemia (TDT), a severe genetic blood disorder.
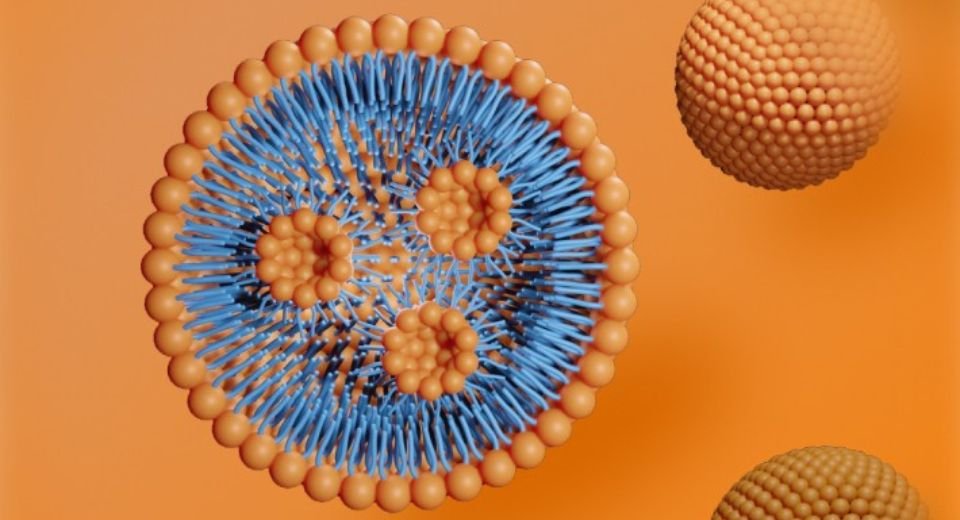
The trial, called YOLT-204 is an in vivo gene-editing therapy, leverages the Shanghai-headquartered company’s proprietary lipid nanoparticles, according to a statement.
It alleviates the imbalance of haemoglobin production and normalises the number of red blood cells in TDT patients. Lipid nanoparticles are used to deliver small molecules, such as drugs, nucleic acids, and herbal compounds.
The trial is a dose-escalation study to preliminarily examine the safety and efficacy of a single-dose regimen with YOLT-204 in TDT. If successful, it may eventually provide an off-the-shelf curative treatment for TDT patients without conditioning chemotherapy and Hematopoietic Stem Cell Transplantation or bone marrow transplant.
Low oxygen levels
Beta thalassemia is a blood disorder that reduces the production of haemoglobin — an iron-containing protein in red blood cells that carries oxygen to cells throughout the body. In people with beta thalassemia, low levels of haemoglobin reduce oxygen levels in the body. Affected individuals also have a shortage of red blood cells or anaemia.
Anaemia can cause pale skin, weakness, fatigue, and more serious complications. People with beta thalassemia are at an increased risk of developing abnormal blood clots.
TDT is a severe genetic blood disorder where regular blood transfusion is required to manage anaemia.
Beta thalassemia is classified into two types depending on the severity of symptoms. Thalassemia major (also known as transfusion-dependent thalassemia or Cooley’s anaemia) and thalassemia intermedia (which is a non-transfusion-dependent thalassemia). Of the two types, thalassemia major is more severe.
Edit intended targets alone
YolTech’s platform has developed proprietary genome editing toolkits and multiple editing strategies. It allows the insertion, deletion and replacement of DNA in a precise manner by editing intended DNA targets alone and avoiding off-target editing.
In pre-clinical models, YOLT-204 showed effective and sustained induction of fetal haemoglobin, suggesting therapeutic potential for TDT. YOLT-204 may also be an effective treatment for patients with sickle cell disease (SCD), according to the statement.
“The initiation of a clinical trial for YOLT-204 represents a significant milestone of gene editing therapy development for TDT and SCD,” said Dr Yuxuan Wu, founder and Chief Executive Officer of YolTech Therapeutics.
The company’s lead candidate targets transthyretin amyloidosis, a rare disease that causes abnormal proteins to build up in the body’s organs and tissues, and is China’s first LNP-mediated in vivo gene editing therapy to enter clinical development.
Healthy version of the gene
YolTech is also advancing therapies for familial hypercholesterolemia and primary hyperoxaluria type 1.
Gene therapy is a technique that uses genes to treat, prevent or cure a disease or medical disorder. Often, gene therapy works by adding new copies of a gene that is broken or by replacing a defective or missing gene in a patient’s cells with a healthy version of that gene.
Both inherited genetic diseases, such as haemophilia and sickle cell disease and acquired disorders, such as leukaemia, have been treated with gene therapy.