HQ Team
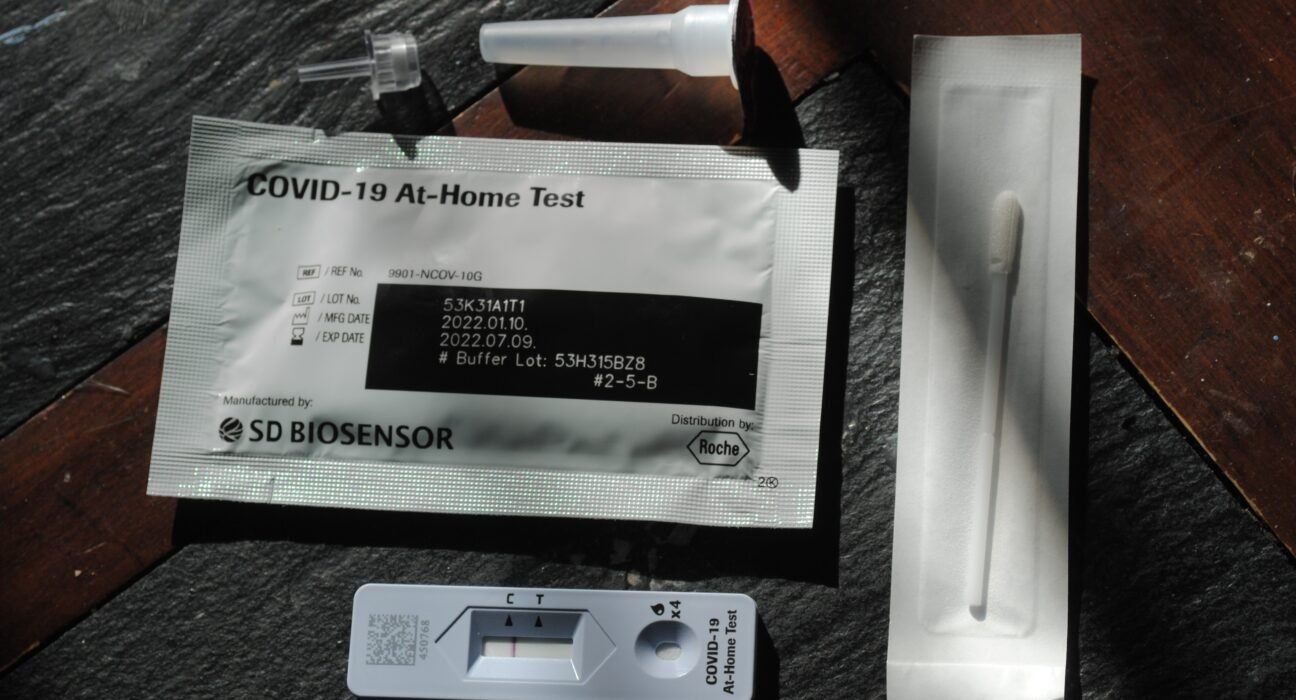
May 5, 2023: The USFDA has warned healthcare providers and consumers from using SD Biosensor Inc. pilot COVID-19 at-home tests due to bacterial contamination.
Direct contact with the contaminated liquid solution, distributed by Roche Diagnostics, could impact the performance of the test, according to an FDA statement.
SD Biosensor, Inc. has initiated a recall for all impacted at-home tests distributed to certain retailers in the United States.
About 500,000 tests were distributed to CVS Health and about 16,000 to Amazon. The FDA is working with Roche Diagnostics to understand how many of those tests were sold to consumers.
None of the impacted lots were distributed through federal testing programs.
Discharge, red eyes
The health watchdog told consumers to watch for signs of bacterial infection caused by exposure to the contaminated liquid solution, such as discharge and red eyes.
“Throw out the entire test kit in the household trash. Do not pour the liquid solution down the drain.”
The liquid solution provided in the affected pilot COVID-19 at-home test kits are contaminated with enterococcus, enterobacter, klebsiella and serratia species.
According to the FDA, individuals performing the self-test may run the risk of direct contact with the contaminated liquid in the tube.
The liquid is in an individual, ready-to-use, pre-filled, and sealed tube.
Weak immune system
The user may inadvertently come in direct contact with a contaminated liquid buffer during opening the tube or handling the open tube, or while performing the test.
Infection from bacteria may cause illness in people with weakened immune systems or those with direct exposure to the contaminated liquid solution through standard handling, accidental spills, or misuse of the product.
To date, the FDA has not received reports of injuries, adverse health consequences, or death associated with using the SD Biosensor pilot COVID-19 at-home test.
The FDA is currently reviewing the test’s recall and is in the process of classifying the recall risk.