HQ Team
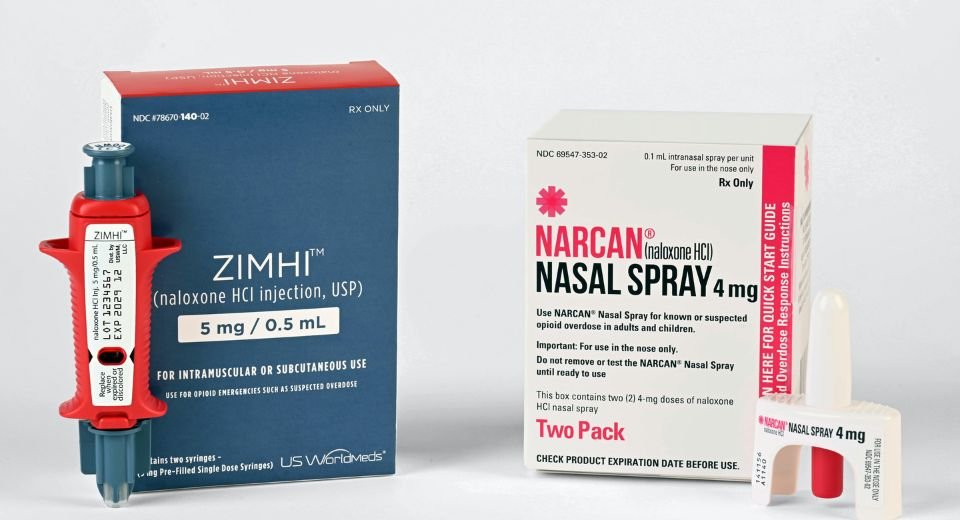
March 29, 2023: US-based Emergent BioSolutions announced that FDA had approved its nasal spray Narcan as an over-the-counter emergency treatment for opioid overuse.
The decision comes at a time when about every eight minutes, a person dies from an opioid overdose, and research shows the epidemic is escalating in the US with the rise in synthetic opioids, according to the company.
Emergent will be able to sell naloxone-based medicine at pharmacies without a prescription in the US.
Narcan spray was developed for use in a community setting, and more than 44 million doses have been distributed since it hit the market in 2016.
The prescription to over-the-counter FDA nod is supported by human factors study data, an updated Drug Facts label, pharmacovigilance data collected from various public sources, and seven years of post-marketing safety data.
Same formulation
The new OTC product will have the same formulation, device design, and original prescription strength (4 mg), which are important features and benefits to help counteract the effects of opioids, including fentanyl, in the community setting, according to the statement.
“Today’s landmark FDA OTC approval for Narcan nasal spray marks a historic milestone,” said Robert G. Kramer, president and CEO of Emergent BioSolutions.
“We have delivered on our commitment to make this important emergency treatment widely accessible, given the alarming rates of opioid overdoses occurring across the country,” he said.
Available by late summer
Emergent expects Narcan nasal spray to be available on US shelves and at online retailers by the late summer to account for manufacturing changes that will be implemented to support nonprescription packaging, as well as supply chain modifications.
“In the meantime, the prescription product will remain in readily available supply through current access channels, including pharmacies through standing order or co-prescription laws and through community distribution,” according to the statement.
Emergent stated that the spray was not a substitute for emergency medical care. Repeat dosing may be necessary.
Drug overdose deaths in the US rose from 2019 to 2021, with more than 106,000 drug overdose deaths reported in 2021, according to the Centers for Disease Control and Prevention.
Fentanyl deaths rise
Deaths involving synthetic opioids other than methadone (primarily fentanyl) continued to rise, with 70,601 overdose deaths reported in 2021.
Those involving stimulants, including cocaine or psychostimulants with abuse potential (primarily methamphetamine), also continued to increase, with 32,537 overdose deaths in 2021.
In 2021, the number of reported deaths involving prescription opioids totalled 16,706.