HQ Team
December 22, 2023: The FDA has seized “thousands of units” of counterfeit weight-loss injection Ozempic which have no information about the drug’s identity, quality or safety.
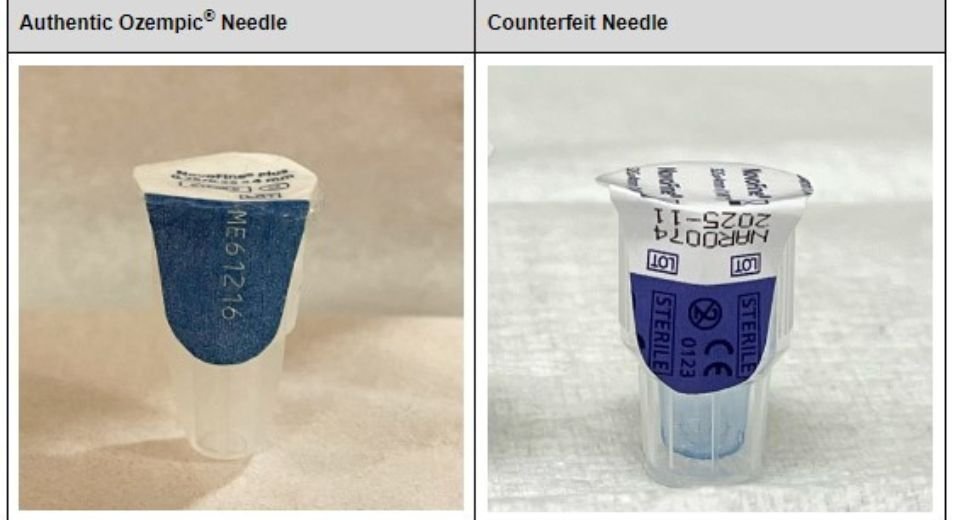
The drug regulator and Novo Nordic, which makes Ozempic (semaglutide), is currently testing the fake products that are sold with counterfeit needles.
“The sterility of the needles cannot be confirmed, which presents an increased risk of infection for patients who use counterfeit products,” according to an FDA statement.
The US drug regulator stated that it was aware of “five adverse events from this lot, none of which are serious and are consistent with known common adverse reactions to authentic Ozempic.”
Those include nausea, vomiting, diarrhoea, abdominal pain and constipation.
Working with Novo Nordisk
The “FDA’s investigation is ongoing, and the agency is working with Novo Nordisk to identify, investigate, and remove further suspected counterfeit semaglutide injectable products found in the US.”
Semaglutide belongs to a class of medications known as glucagon-like peptide-1 (GLP-1) receptor agonists.
It mimics the GLP-1 hormone that is released in the gastrointestinal tract in response to eating.
One role of GLP-1 is to prompt the body to produce more insulin, which reduces blood glucose (sugar).
GLP-1 in higher amounts also interacts with the parts of the brain that reduce appetite and signal a feeling of fullness.
Rise in obese population
The FDA has approved three semaglutide medicines — Ozempic injection, Rybelsus tablets and Wegovy injection.
Patients should be aware that some products sold as ‘semaglutide’ may not contain the same active ingredient as FDA-approved semaglutide products and maybe salt formulations.
Products containing these salts, such as semaglutide sodium and semaglutide acetate, are not safe and effective.
The global market for weight loss products and services is projected to grow at a compound annual growth rate of 8.2% during the forecast period 2021-2026, according to BCC Research.
Weight-loss products
At the end of the forecast period, the market is slated to grow from $254.9 billion in 2021 to reach $377.3 billion by 2026.
The rising prevalence of obesity and disorders such as diabetes, heart disease, stroke, and certain types of cancer is driving the demand for weight loss products.
Also, increasing health awareness among the general population is likely to stimulate and the launch of high-quality weight loss products will drive market growth.