HQ Team
November 1, 2023: GSK, a British pharmaceutical company, will pay $1 billion to Johnson & Johnson for transferring exclusive global rights to develop and commercialise the US company’s hepatitis B therapy.
The therapy, known as JNJ-3989 was initially sold to Johnson & Johnson’s arm Janssen by California-based Arrowhead Pharmaceuticals in 2018.
GSK will get rights and obligations of the existing license agreement between Janssen and Arrowhead, including all remaining financial obligations owed to Arrowhead for JNJ-3989 under the original agreement.
GSK will be responsible for upfront and potential milestone-based payments to both Janssen and Arrowhead totalling approximately $1 billion, according to a J&J statement.
Janssen will continue to be responsible for the ongoing clinical trials of JNJ-3989 at its expense and GSK will be solely responsible for all future development and commercialisation activities.
Help expand GSK hepatitis drug
Arrowhead will receive tiered royalties on net sales under the original agreement. Exclusive rights to the J&J hepatitis B therapy will help expand GSK’s hepatitis B treatment, bepirovirsen, which is currently in late-stage development, according to the statement.
Bepirovirsen, GSK’s investigational antisense oligonucleotide, is meant for the treatment of adult non-cirrhotic patients with chronic hepatitis B on nucleos(t)ide analogue therapy.
According to GSK, there is a high unmet need for chronic hepatitic B patients, with an estimated 300 million people living with the disease and a less than three to seven per cent functional cure rate with current treatment options.
Patients reach functional cures when the hepatitis B virus and viral proteins are at levels low enough to be undetectable in blood and can be controlled by the immune system without medication.
“We are excited to build on promising results already demonstrated with bepirovirsen to investigate a novel sequential regimen with JNJ-3989,” Tony Wood, Chief Scientific Officer, GSK, said.
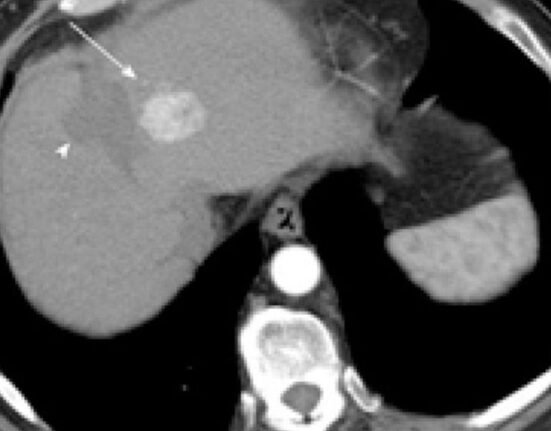
Viral infection of liver
“We believe this approach could redefine the treatment paradigm for chronic hepatitis B by helping even more patients achieve a functional cure.”
Hepatitis B is a viral infection of the liver, caused by the hepatitis B virus, that can cause both acute and chronic liver disease.
Chronic hepatitis B is a long-lasting infection and occurs when the body’s immune system is unable to fight off the virus and it persists in the blood and liver.
Of the 300 million people, only about 10% have a diagnosis and only 5% receive treatment. Even when treated, it can progress to liver complications including cirrhosis and liver cancer, which results in almost a million deaths per year.
JNJ-3989 has the potential to move immediately into a phase II sequential regimen trial with bepirovirsen beginning in 2024, further strengthening GSK’s late-stage pipeline of speciality medicines, according to the GSK statement.