HQ Team
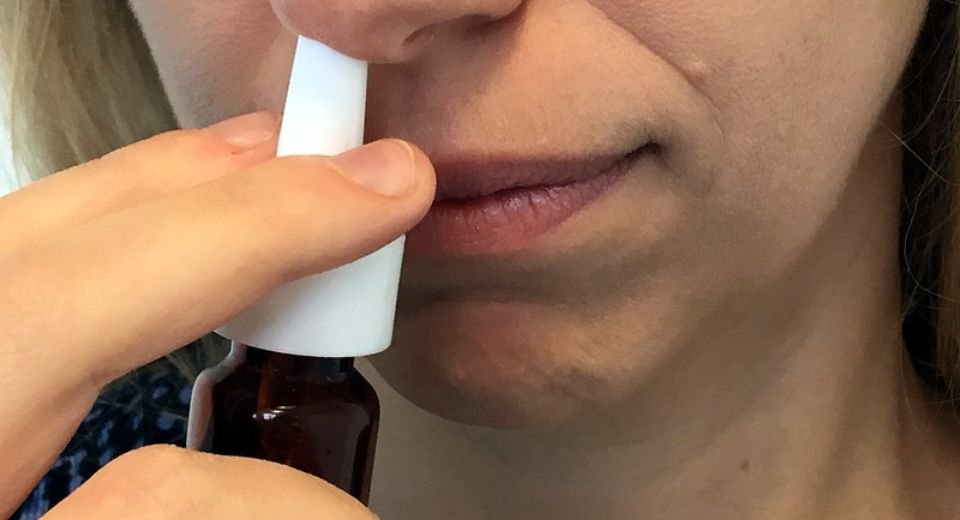
October 21, 2022: A nasal spray drug that treats abnormal heart rhythm has shown promising results in phase 3 trial. The drug, Etripamil, is being tested for treating a medical condition known as paroxysmal supraventricular tachycardia or PSVT.
The trial split 700 patients into two groups. Half were given a nasal spray of Etripamil or a placebo spray without any medical monitoring to see if it could actually cure irregular heartbeats caused by PSVT.
If no relief was found within 10 minutes of using the drug, then patients were advised to administer another dose of the medicine. The researchers said the desired results were achievable in dealing with PSVT within 30 minutes, according to a report by Labiotech.eui.
In the trial, patients who took Epitramil to cure their irregular heartbeats saw relief three times faster than patients who took the placebo. The researchers said that the trial also showed that it was safe to self-administer the drug, Etripamil. The phase 3 trial proceedings were consistent with the phase 2 results.
Currently, treatments for PSVT are very expensive, and this new drug could make treatment more accessible and cost-effective.
This treatment drug for PSVT could be used for further research on how to treat irregular heartbeats and related issues..
Scientists are constantly looking for new ways to improve heart health.
FDA approval for Etripamil
Milestone Pharmaceuticals, a Montreal-based company, is conducting the trials and said that it plans to submit a new drug application to the FDA in mid-2023.
An earlier trial had not thrown any positive results for the condition. In March 2020, in a NODE-301 pivotal trial, etripamil failed to beat placebo in terms of conversion of the arrhythmia supraventricular tachycardia to sinus rhythm in five hours after treatment.
Milestone then changed the trial into a two-part one—NODE 301 and RAPID. The second part, dubbed RAPID, allowed repeat dosing. Milestone also changed the primary endpoint for both parts to conversion within 30 minutes.
A retrospective analysis of the NODE-301 data showed 54% of etripamil patients converted within 30 minutes, compared to 35% of their counterparts in the placebo group. RAPID improved on that result.