HQ Team
May 17, 2024: Johnson & Johnson will acquire Proteologix, Inc., a privately-held biotechnology company, for $850 million in cash, according to a statement.
The transaction is expected to close by the middle of 2024, and has the potential for an additional milestone payment, according to the company statement.
Proteologix focuses on bispecific antibodies for immune-mediated diseases and will expand Johnson & Johnson’s dermatology portfolio with the opportunity to address atopic dermatitis and asthma.
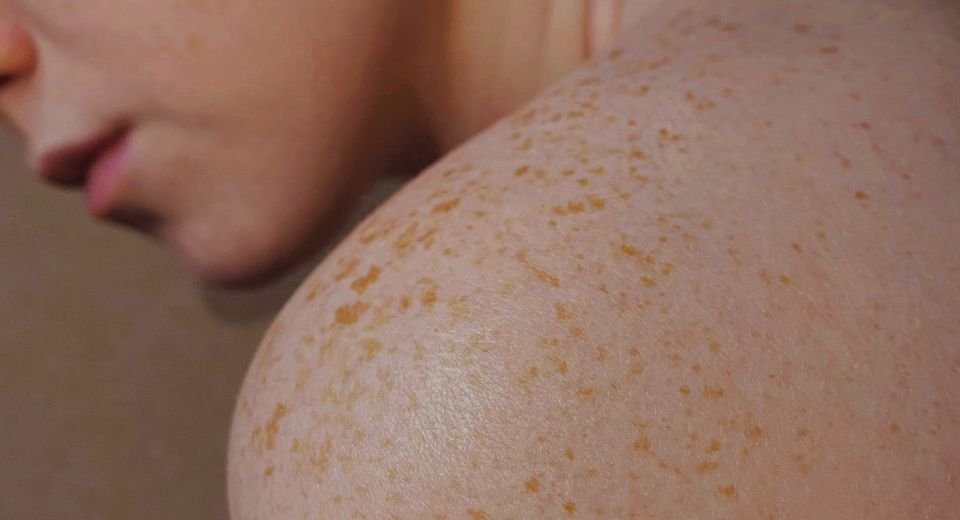
Atopic dermatitis, also known as eczema, is a chronic inflammatory skin disorder affecting more than 102.8 million children and 101.3 million adults worldwide, according to a 2023 study published in the British Journal of Dermatology.
The disease is characterised by an overactive immune system that causes skin inflammation and damage to the skin barrier, leaving it dry, itchy, and prone to subsequent skin infections.
Poor quality of life
The condition can cause a poor quality of life by impacting the ability to interact with family and friends, interrupting sleep due to intense itching and painful skin, and leading to anxiety, stress and depression with an increased risk of suicide.
Proteologix’s PX128, an antibody, is ready to enter phase 1 development for moderate to severe atopic dermatitis and moderate-to-severe asthma, and another antibody PX130 is in the preclinical development stage.
“Atopic dermatitis is the most common inflammatory skin disease, impacting more than 100 million adults worldwide,” said David Lee, Global Immunology Therapeutic Area Head, Johnson & Johnson Innovative Medicine. “About 70% of patients using existing standard-of-care therapies do not reach remission.
“Current advanced therapies for atopic dermatitis either target a single pathway and have limited efficacy or are more broadly immunosuppressive, resulting in significant safety concerns,” he said. “We see an opportunity for best-in-disease efficacy for PX128 and PX130.”
Asthma is a chronic lung disease affecting 262 million people globally across all ages, sexes, ethnicities and races. Its symptoms include coughing, wheezing, and difficulty in breathing caused by inflammation and narrowing of the airways.
Genetic, environmental
Genetic and environmental factors have been linked to asthma development, and triggers, such as allergens and infections can start or worsen asthma symptoms. Severe asthma attacks can be life-threatening and may require emergency room visits and hospitalisations.
While asthma can be managed by medications and avoiding triggers for most patients, there is currently no cure, and the burden of asthma on patients’ quality of life and health resource utilisation remains high.
“Integrating Proteologix bispecific antibodies into our pipeline is an important first step in fulfilling our commitment to people living with atopic dermatitis,” said Candice Long, Worldwide Vice President of immunology, at Johnson & Johnson.
“We plan to continue expanding our reach and impact for people living with a wide variety of immune-mediated diseases, leveraging more targeted options for them to reach durable, symptom-free remission.”