HQ Team
May 13, 2024: Merck has discontinued another cancer experimental immunotherapy treatment in patients with a severe form of melanoma due to side effects.
Merck was testing the combination drug, vibostolimab, an anti-TIGIT antibody, and pembrolizumab (Keytruda) Merck’s anti-PD-1 therapy, compared to Keytruda alone, as adjuvant treatment for patients with resected high-risk melanoma.
An independent Data Monitoring Committee (DMC) has recommended unblinding the study and patients receiving the vibostolimab and pembrolizumab coformulation be offered the option to be treated with Keytruda monotherapy. After a proper investigation the data will be communicated to regulatory agencies.
Keytruda
In the U.S., Keytruda has two approved indications in melanoma: for the treatment of patients with unresectable or metastatic melanoma, and for the adjuvant treatment of adult and pediatric (12 years and older) patients with stage IIB, IIC, or III melanoma following complete resection.
Merck said it still had an extensive clinical development program evaluating the vibostolimab-Keytruda combination. The program includes the ongoing Phase 3 V940-001 study, in collaboration with Moderna. The combination of Moderna’s experimental mRNA vaccine with Keytruda showcased a 44% reduction in the risk of death or recurring disease compared to those not receiving the vaccine.
Earlier, Merck Group’s investigational drug Keytruda, to treat gastric cancer, has failed to meet one of its two set goals. The combination treatment had earlier failed to significantly slow disease progression in lung cancer patients in a late-stage study last year.
Melanoma
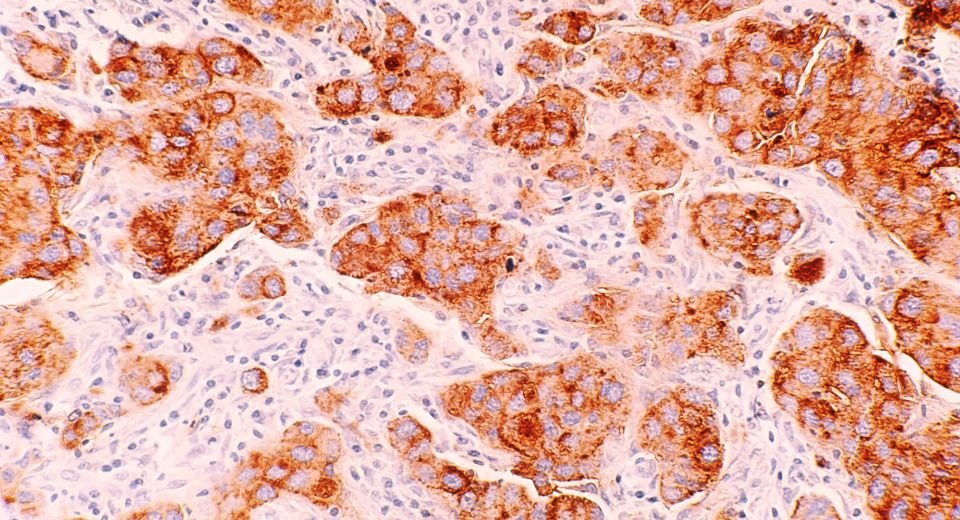
Melanoma, the deadliest form of skin cancer, poses a significant public health concern. The rates of melanoma have been rising over the past few decades, with nearly 325,000 new cases diagnosed worldwide in 2020. In 2023, nearly 187,000 Americans were expected to be diagnosed with melanoma, with over 97,600 cases identified as invasive, according to the Melanoma Research Foundation.