HQ Team
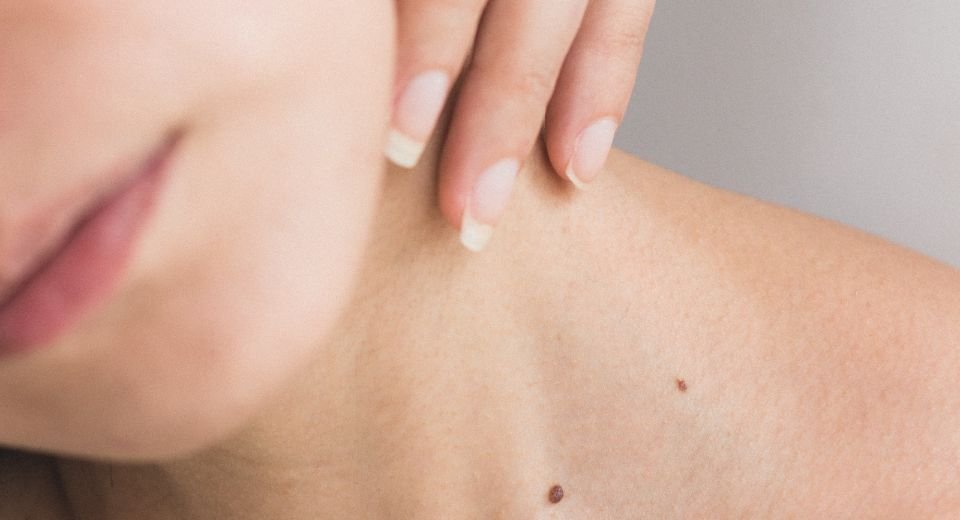
December 23, 2023: Moderna unveiled a skin cancer vaccine that demonstrated promising results in recent clinical trials. The vaccine, administered in conjunction with Merck’s immunotherapy drug, Keytruda, exhibited a statistically significant improvement in survival rates for 157 patients with advanced melanoma, according to the Hackensack Meridian John Theurer Cancer Center in New Jersey, a key participant in the trials.
In the study, the combination of Moderna’s mRNA vaccine and Keytruda showcased a remarkable 44% reduction in the risk of death or recurring disease among patients who had previously undergone surgery to remove their cancer. The results suggest a potential game-changer in the treatment landscape for melanoma, a fast-growing and deadly form of skin cancer affecting a significant number of people.
Keytruda, a checkpoint inhibitor, blocks an enzyme that cancer cells use to evade the immune system. While Keytruda is effective in certain mutagenic cancers, including melanoma, it can lose its impact as the cancer mutates and becomes resistant to immune responses. The combination of Moderna’s experimental mRNA vaccine with Keytruda showcased a 44% reduction in the risk of death or recurring disease compared to those not receiving the vaccine.
Dr. Andrew Pecora, an oncologist and researcher at the Hackensack Meridian John Theurer Cancer Center, expressed excitement, describing the vaccine as “truly game-changing, groundbreaking stuff.” He highlighted the limitations of current immunotherapy, stating that melanomas often remain undetected by the immune system in about half of cancer patients.
Tailored antitumor response
The Moderna vaccine aims to revolutionize the immune system’s ability to recognize and combat melanoma by offering a personalized approach. As each tumor in a patient is unique, a personalized mRNA vaccine tailored to the specific DNA changes in their cancer cells can be effective Personalized cancer vaccines are designed to prime the immune system so that a patient can generate a tailored antitumor response specific to their tumor mutation signature. This approach addresses one of the limitations of current immunotherapy, offering a more targeted and effective solution.
FDA approval awaited
As the vaccine enters Phase 3 trials, researchers are optimistic about its potential approval by the FDA. The trial participants reported no side effects beyond those associated with immunotherapy.
“The results of this randomized Phase 2b trial are exciting for the field. These data provide the first evidence that we can improve on the rates of recurrence-free survival achieved by PD-1 blockade in resected high-risk melanoma. These findings also provide the first randomized evidence that a personalized neoantigen approach may be beneficial in melanoma,” said Jeffrey S. Weber, MD, PhD, principal investigator of the study and Deputy Director of the Perlmutter Cancer Center at NYU Langone. Dr. Weber is a paid consultant for Merck and Moderna.
Melanoma, the deadliest form of skin cancer, poses a significant public health concern. The rates of melanoma have been rising over the past few decades, with nearly 325,000 new cases diagnosed worldwide in 2020. In 2023, nearly 187,000 Americans were expected to be diagnosed with melanoma, with over 97,600 cases identified as invasive, according to the Melanoma Research Foundation. The promising results from Moderna’s vaccine offer a ray of hope in the ongoing battle against this aggressive form of cancer.