HQ Team
November 3, 2024: The National Instituted of Health, US, has started a mid-stage clinical trial to examine the safety and acceptability of a rectal microbicide douche containing the antiretroviral drug tenofovir to prevent HIV infection spread among men.
The “on-demand” approach involves using the microbicide before a potential exposure from receptive anal intercourse, according to an NIH statement.
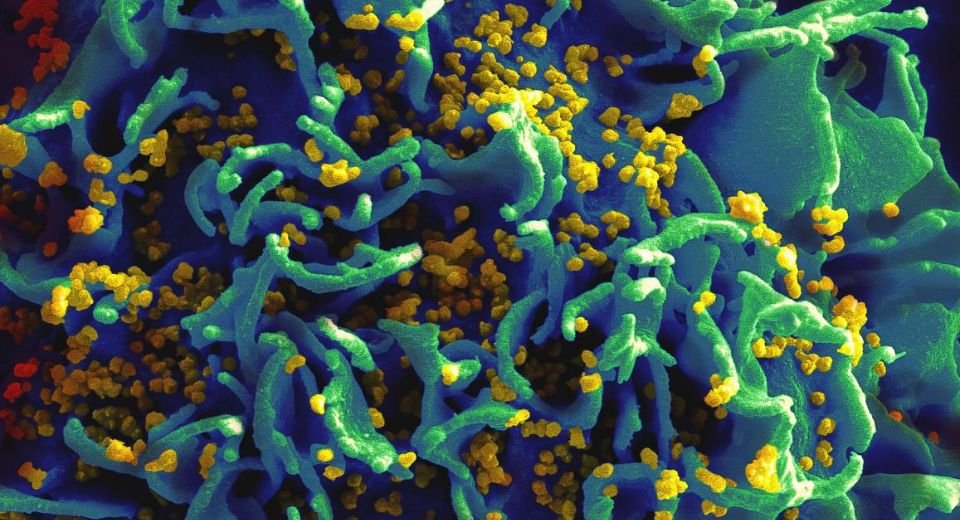
Human immunodeficiency virus (HIV) is an infection that attacks the body’s immune system, specifically the white blood cells called CD4 cells. HIV destroys these CD4 cells, weakening a person’s immunity against opportunistic infections, such as tuberculosis, fungal infections, severe bacterial infections, and some cancers.
HIV infection can be diagnosed using simple and affordable rapid diagnostic tests, and self-tests.
Oral pills, vaginal ring
Several forms of HIV pre-exposure prophylaxis (PrEP) are in use in the US and globally — daily oral pills, long-acting injections, and a monthly vaginal ring.
The Centers for Disease Control and Prevention recommends gay, bisexual and other men who have sex with men who meet certain criteria can take “on-demand” oral PrEP around the time of sex to prevent HIV acquisition.
There is insufficient evidence to support its use in other populations.
Rectal microbicides are another HIV prevention method being explored for use in an “on-demand” manner to expand the choices available to eligible people who engage in receptive anal intercourse and who stand to benefit from using PrEP.
The clinical trial will enrol about 150 adults assigned male at birth who have regular experience using an unmedicated rectal douche before receptive anal intercourse.
Microbicide douche
Trial participants will each receive “on-demand” tenofovir rectal microbicide douche during one two-month period and on-demand oral PrEP with tenofovir disoproxil fumarate and emtricitabine in another two months.
The study will also assess participants’ experience using their assigned PrEP method, including measures of acceptability, adherence, and method preference. It will take place at eight sites in the United States.
The incidence of HIV is decreasing slowly in the US. Still, 67% of US HIV diagnoses from 2018-2022 were among gay, bisexual, and other men who have sex with men, pointing to the need for expanded HIV prevention options.
The mid-stage study is sponsored by NIH’s National Institute of Allergy and Infectious Diseases (NIAID) and implemented through the NIH-funded HIV Prevention Trials Network.
NIAID conducts and supports research at NIH, throughout the United States, and worldwide, to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses.