HQ Team
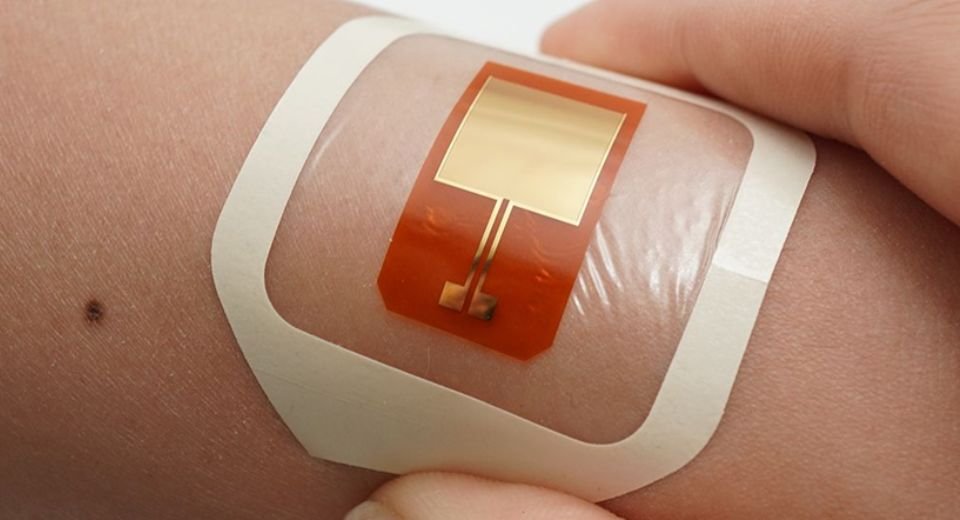
October 2, 2024: A device named Bioelectronic Localized Antimicrobial Stimulation Therapy (BLAST) has been developed to prevent skin infections by using harmless electrical currents to target harmful bacteria. This innovative patch, has been made by the Bozhi Tian (UChicago) and Gürol Süel (UC San Diego) labs. It adheres to the skin, delivers a weak electrical current that disrupts the activity of disease-causing bacteria.
How BLAST works
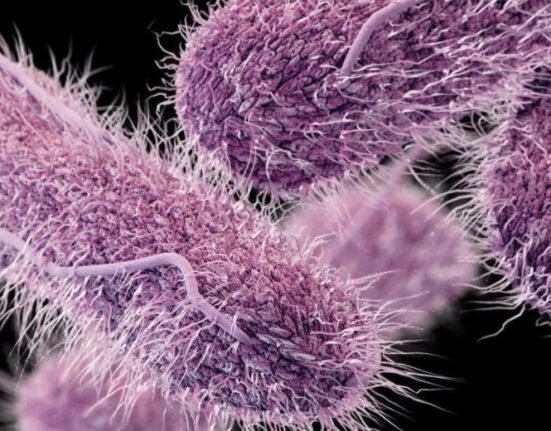
In recent studies conducted on pig skin, researchers found that the device effectively reduced the presence of Staphylococcus epidermidis, a common bacterium found on healthy skin that can lead to infections when it enters the body through contaminated medical devices. The device operates by delivering 10-second electrical pulses every ten minutes, significantly decreasing biofilm coverage and bacterial counts by nearly tenfold compared to untreated skin
The key to BLAST’s effectiveness lies in its ability to create an acidic environment under the patch, which enhances the bacteria’s response to electrical stimulation. This process inhibits the bacteria’s ability to form biofilms, which are protective layers that allow them to adhere to surfaces and resist treatment.
The electrical pulses used are similar in voltage to those delivered by pacemakers, ensuring safety while achieving significant antimicrobial effects.
In preclinical tests, the electroceutical patch demonstrated remarkable results, achieving nearly a tenfold reduction in bacterial colonization on pig skin. “We discovered action potentials in bacterial biofilms almost ten years ago and since then we have worked to show that bacteria, which are typically not thought of as excitable, are indeed excitable and even perform functions similar to neurons in the brain,” said Gürol Süel, a professor in the UC San Diego School of Biological Sciences.
Future implications
The implications of this technology are vast. If proven effective in further studies involving living animals and eventually humans, BLAST could serve as an alternative to antibiotics, potentially reducing the risk of antibiotic resistance—a critical public health concern. Drug-resistant infections are estimated to increase 70 per cent by 2050, making the development of this device and its accompanying treatments essential for ensuring effective infection control in an increasingly resistant microbial landscape.
Researchers are optimistic about its application in clinical settings, particularly for patients with chronic wounds or those requiring catheterization.
The research team plans to explore whether other bacterial species respond similarly to electrical stimulation. This could broaden the clinical applications of BLAST beyond just S. epidermidis. If successful, the device could be available for public use within five years.