HQ Team
March 12, 2024: Pfizer Inc., announced that a late-stage study on its Adcetris drug, for treating the most common lymphoma, yielded positive results and improved overall survival rates.
“Positive outcomes were also observed in key secondary endpoints, including progression-free survival and overall response rate,” according to an emailed statement.
The safety and tolerability of Adcetris in the trial were consistent with what has been previously presented for patients with relapsed/refractory diffuse large B-cell lymphoma.
The combination therapy of Adcetris and two other drugs was “statistically significant” compared to a placebo in extending survival in patients with diffuse large B-cell lymphoma, according to the Pfizer statement.
Seven indications
The therapy has been approved for seven indications in the US with more than 55,000 patients treated since its first US approval in 2011.
More than 140,000 patients have been treated globally. Pfizer plans to share the data with the US Food and Drug Administration to potentially support regulatory filing in the US, potentially leading to the eighth approval for Adcetris.
Full data will be submitted for presentation at an upcoming medical meeting.
Pfizer and Takeda are jointly developing Adcetris. Under the terms of the collaboration agreement, Pfizer has US and Canadian commercialization rights, and Takeda has rights to commercialize Adcertis in the rest of the world.
Pfizer and Takeda are funding joint development costs for the drug on an equal basis, except in Japan where Takeda is solely responsible for development costs.
‘Encouraging’
“The results are particularly encouraging because the study evaluated heavily pre-treated patients, including some who received prior CAR-T therapy,” said Roger Dansey, MD, Chief Development Officer, Oncology, Pfizer.
“This is the third Phase 3 study in a type of lymphoma to demonstrate an overall survival benefit for an Adcetris combination.
“Based on the strong results from,” the trial “we’re excited that Adcetris could address an area of high unmet need in patients with relapsed or refractory,” diffuse large B-cell lymphoma.
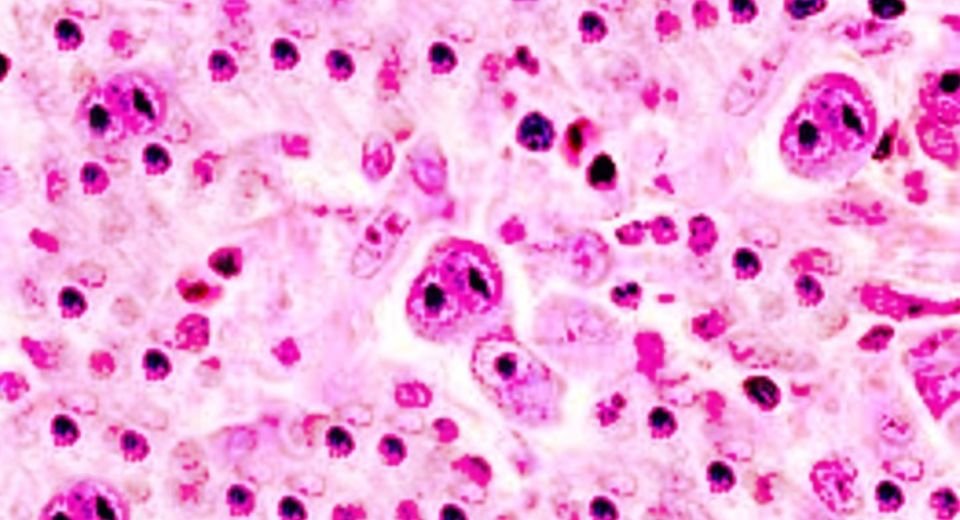
Diffuse large B-cell lymphoma is the most frequent type of lymphoma and is an aggressive, difficult-to-treat disease. More than 25,000 cases of the disease are diagnosed each year in the US, accounting for more than 25% of all lymphoma cases.
The disease can develop spontaneously or due to chronic lymphocytic lymphoma/small lymphocytic lymphoma, follicular lymphoma, or marginal zone lymphoma.
Up to 40% of patients relapse or have refractory disease after frontline treatment.