HQ Team
December 21, 2023: Sweden’s Calliditas Therapeutics has got the USFDA green light for its oral drug to treat loss of kidney function in adults with primary immunoglobulin A nephropathy (IgAN) and at risk of disease progression.
IgAN is a kidney disorder in which antibodies (called IgA) build up in kidney tissue. Nephropathy is damage, disease, or other problems of the kidney. IgAN is also called Berger disease.
The damage may cause few or no symptoms. Blood in the urine is the most common symptom. The condition is diagnosed by blood and urine tests.
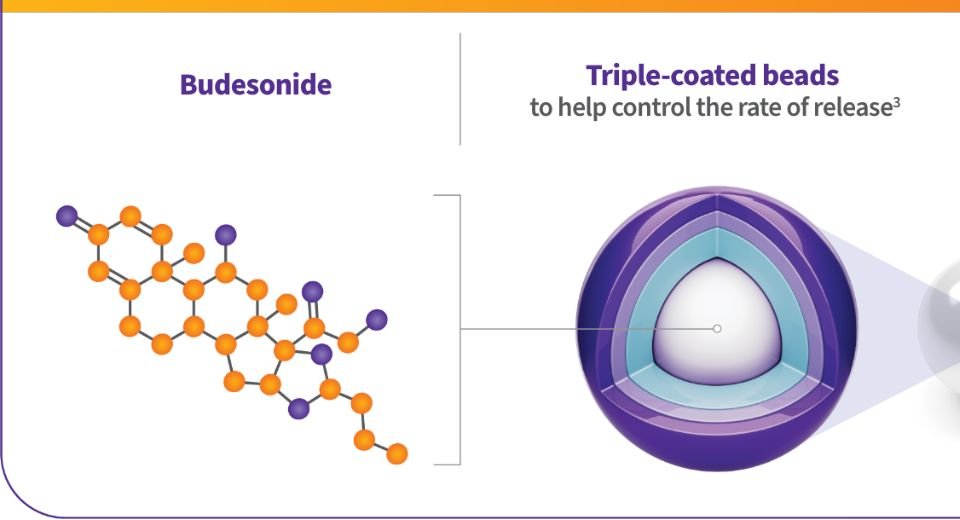
The drug Tarpeyo, a budesonide delayed-release capsule, was first approved in December 2021 under accelerated approval of the FDA.
Filter waste from blood
The medicine was fully approved by the regulator after the final stage of clinical trials proved positive toward the primary efficacy endpoint, which was a time-weighted average of estimated glomerular filtration rate (eGFR) over two years.
Glomerular filtration rate is a test used to check how well the kidneys are working. Glomeruli are the tiny filters in the kidneys that filter waste from the blood.
“The evidence of sustained reductions in proteinuria and a clinically significant reduction in the loss of eGFR, which can help slow the progression towards dialysis or transplant care, highlights the potential of Tarpeyo as a disease-modifying agent in IgAN,” said Richard Lafayette, MD, FACP, Stanford Healthcare.
IgAN is not just a kidney disease, according to the National Kidney Foundation. It is a response of one’s immune system to outside viruses.
‘Critical step’
The immune response is what affects your kidneys. The immune response releases immunoglobulin A, a protein that helps your body fight infections.
“This first-ever IgAN treatment to get full approval based on kidney function represents a beacon of hope for the entire IgA nephropathy community and signifies a critical step forward in the battle against IgAN,” said Bonnie Schneider, director and co-founder of the IgAN Foundation.
It validated a nearly 20-year journey since founding this volunteer-run organization to raise awareness and promote research for IgAN, she said.