HQ Team
December 8, 2023: The USFDA has approved two treatments for sickle cell disease, one of them the first in the US based on gene-editing technology.
The regulator cleared Casgevy, developed by Vertex Pharmaceuticals and CRISPR Therapeutics and bluebird bio’s Lyfgenia for treatment in people aged 12 years and older.
“Casgevy, is the first FDA-approved treatment to utilize a type of novel genome editing technology, signalling an innovative advancement in the field of gene therapy,” according to a statement.
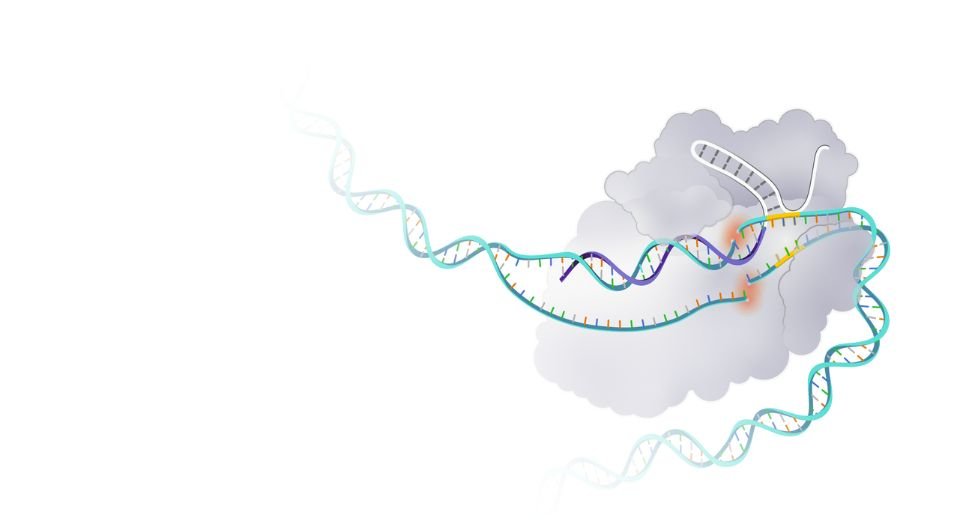
Casgevy therapy involves CRISPR/Cas9, a type of genome editing technology. Patients’ hematopoietic (blood) stem cells are modified by genome editing using CRISPR/Cas9 technology.
Cut DNA
CRISPR/Cas9 can be directed to cut DNA in targeted areas, enabling the ability to accurately edit (remove, add, or replace) DNA where it was cut.
The modified blood stem cells are transplanted back into the patient where they engraft, or attach and multiply, within the bone marrow and increase the production of fetal haemoglobin (HbF), a type of haemoglobin that facilitates oxygen delivery. In patients with sickle cell disease, increased levels of HbF prevent the sickling of red blood cells.
Sickle cell disease is a group of inherited blood disorders affecting approximately 100,000 people in the US. It is most common in African Americans and, while less prevalent, also affects Hispanic Americans.
The primary problem in sickle cell disease is a mutation in haemoglobin, a protein found in red blood cells that delivers oxygen to the body’s tissues. This mutation causes red blood cells to develop a crescent or “sickle” shape.
Restrict blood flow
These sickled red blood cells restrict the flow in blood vessels and limit oxygen delivery to the body’s tissues, leading to severe pain and organ damage called vaso-occlusive events or vaso-occlusive crises.
The recurrence of these events or crises can lead to life-threatening disabilities or early death.
“Gene therapy holds the promise of delivering more targeted and effective treatments, especially for individuals with rare diseases where the current treatment options are limited,” said Nicole Verdun, MD, director of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research.
Lyfgenia is a cell-based gene therapy. Lyfgenia uses a gene delivery vehicle for genetic modification and is approved for the treatment of patients 12 years of age and older with sickle cell disease and a history of vaso-occlusive events.
With Lyfgenia, the patient’s blood stem cells are genetically modified to produce a gene-therapy-derived haemoglobin.
Patient’s stem cells
This functions similarly to haemoglobin A, the normal adult haemoglobin produced in persons not affected by sickle cell disease.
Both products are made from the patient’s blood stem cells, which are modified and given back as a one-time, single-dose infusion as part of a blood stem cell transplant.
“These approvals represent an important medical advance with the use of innovative cell-based gene therapies to target potentially devastating diseases and improve public health,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.