HQ Team
April 14, 2023: New safety warnings will be required on prescription labels for opioid pain relievers to prevent addiction and overdose, the FDA stated.
The new warnings must provide information about the amount of dosage and administration and the eligibility to take opioid pain drugs, according to an FDA statement.
The updated labels should have a warning about increased sensitivity to pain, it stated.
“In assessing the severity of pain, discuss with the patient the impact of the pain on their ability to function and their quality of life,” according to the statement.
“Assessment of pain should consider both the cause of pain and individual patient factors.”
Serious risks
Opioid pain medicines are a class of powerful pain medicines prescribed to treat pain that does not respond well to other treatments.
They activate an area of nerve cells in the brain and body that block pain signals. These medicines have benefits when used appropriately but have serious risks, including misuse and abuse, addiction, overdose, and death.
If alternative treatment options are insufficient, the healthcare professional should prescribe the lowest effective dose of an immediate-release opioid for the shortest duration to reduce the risks.
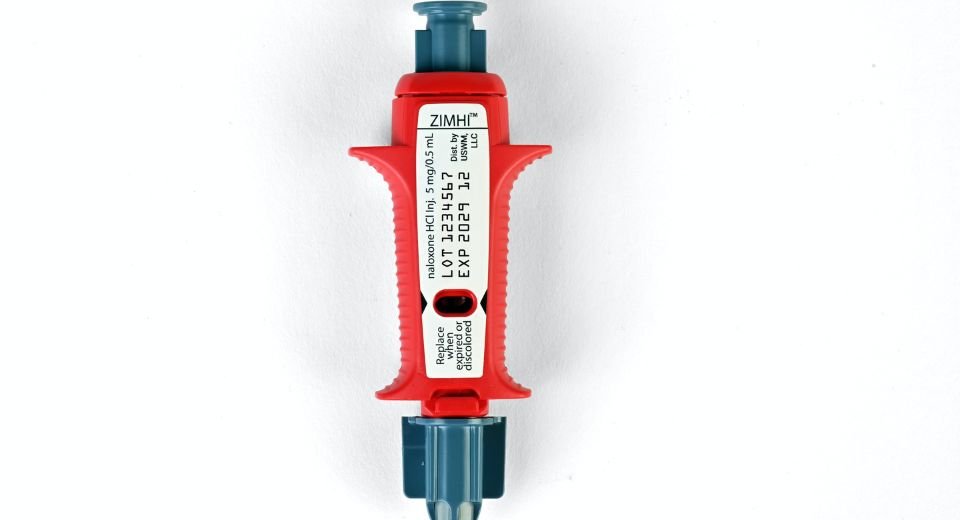
According to the US Food and Drug Administration, patients must be encouraged to discuss the availability of naloxone, and caregivers should consider prescribing it to those at increased risk of overdose.
The health authority warned medical professionals to be aware that the symptoms of opioid-induced hyperalgesia, OIH — a condition where opioids cause an increase in pain called hyperalgesia or increased sensitivity to pain called allodynia.
The symptoms “are distinct from opioid tolerance and withdrawal and can be difficult to recognize.”
Limitations on use
The updates stated that immediate-release opioids should not be used for an extended period unless the pain remains severe enough. This may include pain occurring with many surgical conditions or musculoskeletal injuries.
The FDA, on the extended-release and long-acting opioid treatment, stated that they are reserved for severe and persistent pain.
The usage requires an extended treatment period with a daily opioid pain medicine, for which alternative treatment options are inadequate.
Information in the Boxed Warning, FDA’s most prominent warning, for all immediate-release and extended-release and long-acting release opioid pain medicines will be updated and reordered.
The move will elevate the importance of warnings concerning life-threatening respiratory depression and risks associated with opioid pain medicines in conjunction with benzodiazepines or other medicines that depress the central nervous system.
Naloxone
The FDA also requires updates to the existing patient medication guides to help educate patients and caregivers about these risks.
Examples of common opioid pain medicines include codeine, hydrocodone, hydromorphone, morphine, oxycodone, oxymorphone, fentanyl, buprenorphine, and tramadol.
The FDA advised patients to take their opioid medicines exactly as prescribed, store them securely and not share them with anyone else.
Unused or expired medicines must be immediately disposed of. Patients were advised to seek emergency medical help on signs of respiratory problems, which can be life-threatening.
Signs and symptoms include serious slowed, shallow, or difficult breathing, severe sleepiness and inability to respond or wake up.
Patients must talk to healthcare professionals about the benefits of naloxone, which can reverse an opioid overdose, and how to obtain it.