HQ Team
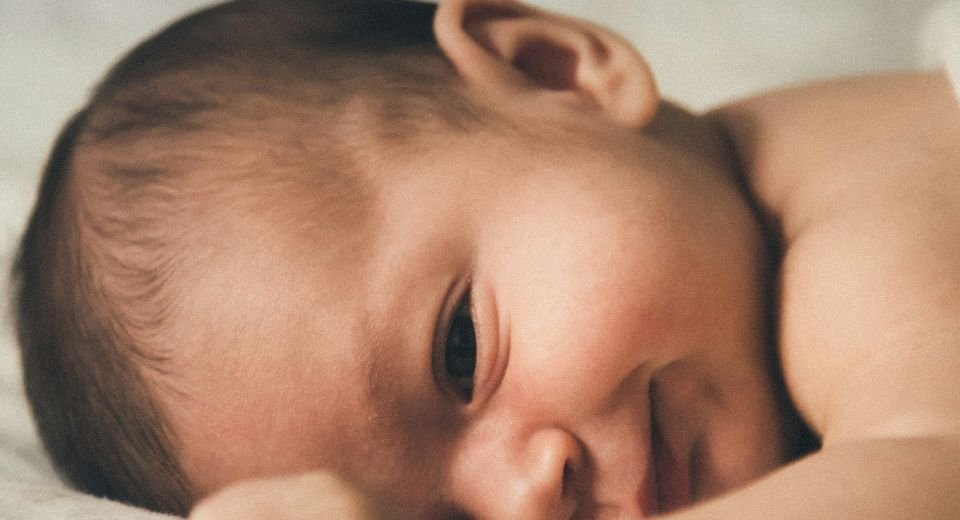
May 15, 2024: An 18-month-old baby girl, Opal Sandy, who was born deaf got back her hearing ability after gene therapy — the youngest child to receive such treatment.
Opal was born completely deaf because of a rare genetic condition, auditory neuropathy, caused by the disruption of nerve impulses travelling from the inner ear to the brain.
Within four weeks of having the gene therapy infusion to her right ear, Opal responded to sound, even with the cochlear implant in her left ear switched off.
The global gene therapy trial, which started in May 2023, aimed to show whether gene therapy can provide hearing for children born with auditory neuropathy.
Opal underwent gene therapy at Addenbrooke’s Hospital in Cambridge when she was 11 months old.
‘Future of audiology’
“Gene therapy has been the future of otology and audiology for many years and I’m so excited that it is now finally here,” Manohar Bance, the chief investigator of the trial said.
Manohar works at the Department of Clinical Neurosciences at the University of Cambridge and is an ear surgeon at Cambridge University Hospitals NHS Foundation Trust.
“These results are spectacular and better than I expected. This is hopefully the start of a new era for gene therapies for the inner ear and many types of hearing loss.”
Opal Sandy, from Oxfordshire, is the first patient treated in a global gene therapy trial. She is the first British patient in the world, and the youngest child to receive this type of treatment, according to a university statement.
‘Dada, bye-bye’
Clinicians noticed a continuous improvement in Opal’s hearing in the weeks afterwards. At 24 weeks, they confirmed Opal had close to normal hearing levels for soft sounds, such as whispering, in her treated ear.
Now 18 months old, Opal can respond to her parents’ voices and can communicate words such as “Dada” and “bye-bye.”
Auditory neuropathy can be due to a variation in a single gene, called the OTOF gene. The gene produces a protein called otoferlin, which is needed to allow the inner hair cells in the ear to communicate with the hearing nerve.
About 20,000 people across the UK, Germany, France, Spain, and Italy are deaf due to a mutation in the OTOF gene.
Children with a variation in the OTOF gene often pass the newborn screening, as the hair cells are working but not talking to the nerve.
Speech delay
It means this hearing loss is not commonly detected until children are two or three years of age – when a speech delay is likely to be noticed.
Professor Bance said: “We have a short time frame to intervene because of the rapid pace of brain development at this age. Delays in the diagnosis can also confuse families as the many reasons for delayed speech and late intervention can impact a child’s development.”
“This trial shows gene therapy could provide a future alternative.”
Mutations in the OTOF gene can be identified by standard genetic testing. Opal was identified as being at risk as her older sister has the condition. This was confirmed by genetic test results when she was three weeks old.
Opal was given an infusion containing a harmless virus. It delivered a working copy of the OTOF gene injected in the cochlea during surgery under general anaesthesia.
‘Less follow-up’
During surgery, while Opal was given gene therapy in the right ear, a cochlear implant was fitted in her left ear.
Dr Richard Brown, Consultant Paediatrician at CUH, an investigator on the trial, said: “It is likely that in the long run such treatments require less follow up so may prove to be an attractive option, including within the developing world.
“Follow-up appointments have shown effective results so far with no adverse reactions and it is exciting to see the results to date.”
The trial is being funded by Regeneron. Patients from the US, UK and Spain are being enrolled.
Adaption to speech
Patients received a low dose of the harmless virus in on of the ears during the first phase.
During the second phase, they are expected to use a higher dose of gene therapy in one ear only, following the proven safety of the starting dose.
The third phase will look at gene therapy in both ears with the dose selected after ensuring the safety and effectiveness in parts one and two.
Follow-up appointments will continue for five years for enrolled patients, to judge how patients adapt to understand speech in the longer term.