HQ Team
March 28, 2024: US regulator, the FDA has approved Gilead Sciences, Inc.’s supplemental new drug application for treating chronic hepatitis B virus in pediatric patients six years of age and older.
The drug Vemlidy (tenofovir alafenamide) 25 mg tablets, taken as a once-daily treatment, was approved for adult patients with compensated liver disease in 2016 and six years later the regulator gave its nod for patients 12 years of age and above.
The regulator allows companies to make changes to drugs or their labels after they have been approved. To change a label, market a new dosage or strength of a drug, or change the way it manufactures a drug, a company must submit a supplemental new drug application (sNDA).
The supplement type refers to the kind of change that was approved by the FDA. This includes changes in manufacturing, patient population, and formulation.
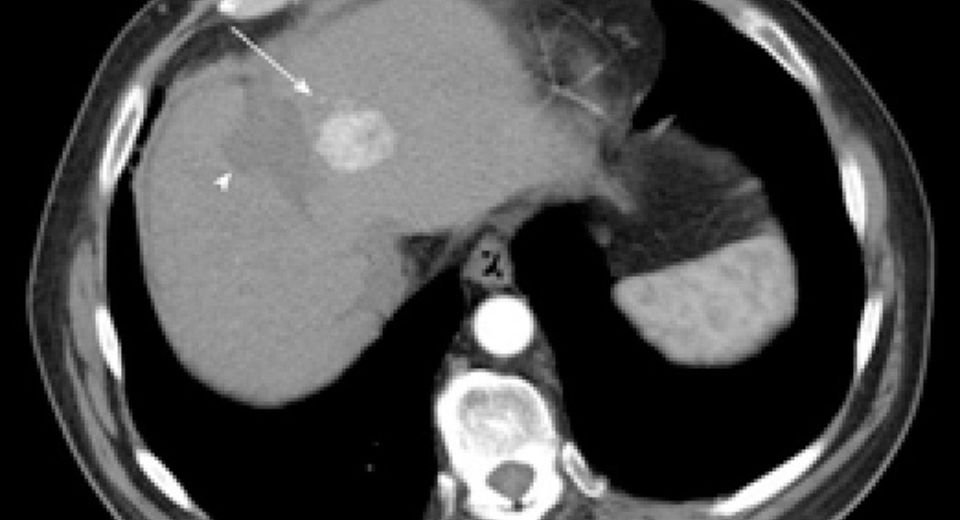
Liver cancer
“Chronic hepatitis B can have a significant and lasting impact on the health of children. If left untreated, hepatitis B can lead to liver cirrhosis and liver cancer,” said Chaun-Hao Lin, MD, Associate Professor of Clinical Pediatrics Krek School of Medicine of USC.
“As a clinician, I am well aware of the critical importance of promptly treating this disease to avoid possible complications and liver damage. The clinical trial demonstrated that tenofovir alafenamide may represent an effective treatment option for children as young as six years old affected by this chronic disease.”
Vemlidy’s approval for treating patients six years and older was based on a phase 2 clinical trial comparing treatment with Vemlidy 25 mg to placebo among 18 treatment-naïve and treatment-experienced patients aged six to less than 12 years weighing at least 25 kg.
Participants in the Vemlidy group and in the placebo group who switched to open-label Vemlidy after 24 weeks “demonstrated progressive increases in the rates of virological suppression through Week 96 overall and within both study cohorts (children and adolescents),” according to a company statement.
‘Safety, efficacy’
“The expanded indication for Vemlidy for the treatment of children as young as six years old is a testament to the safety, tolerability and efficacy profile of this therapy,” said Frank Duff, MD, Senior Vice President, Virology Therapeutic Area Head, Gilead Sciences.
Vemlidy has a boxed warning on its product label regarding post-treatment severe acute exacerbation of hepatitis B.
Hepatitis B is a serious disease that attacks the liver and can cause chronic (lifelong) infection, cirrhosis of the liver, liver cancer, and death in up to a third of patients.
Hepatitis B is spread through infected blood or body fluids, sexual contact, injection drug use, or perinatally from mother to child. Early symptoms may include loss of appetite, fever, generalized aches and pains, fatigue, itching, urticaria (hives), and joint pain.
The disease is often asymptomatic, which may lead to undiagnosed individuals. Later symptoms may include nausea and vomiting, halitosis (bad breath), dark brown urine, jaundice (yellowing of the skin and eyes), and right-sided abdominal pain (especially with external pressure or palpitation).
Gilead operates in more than 35 countries worldwide, with headquarters in Foster City, California, US.