HQ Team
September 18, 2023: German biotechnology company, BioNTech SE announced a $ 90 million program in partnership with the Coalition for Epidemic Preparedness Innovations (CEPI) to develop a vaccine for mpox.
The mpox vaccine program, labelled as BNT166, is part of BioNTech’s efforts to develop a prophylactic vaccine for a range of infectious diseases with a high medical need, according to a BioNTech statement.
Since the eradication of smallpox in 1980, the global population-level immunity against the orthopoxvirus viral family, including mpox, has been waning.
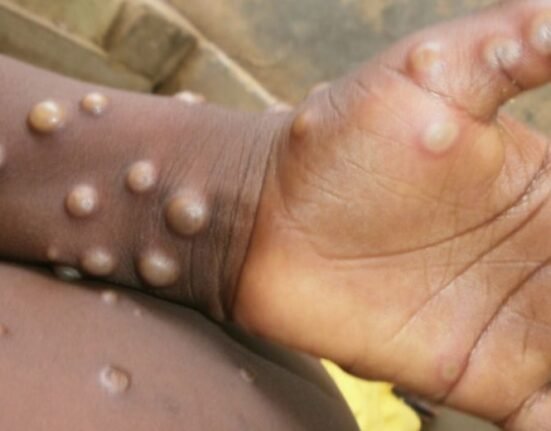
Mpox, an infectious disease that can lead to severe, life-threatening complications, gained global attention in May 2022 with an increasing number of cases that developed into an international outbreak.
Smallpox, cowpox
Orthopoxvirus is a large double-stranded DNA virus that infects mammals and humans. There are 12 species in this family. Diseases associated with this type include smallpox, cowpox, horsepox, camelpox, and mpox.
“Although vaccines against members of the orthopoxvirus family are currently available, there is “a high need for a mpox vaccine broadly available especially in endemic regions.,” according to the statement.
BioNTech is aiming to develop a prophylactic mRNA-based mpox vaccine with a “favourable safety profile that can be manufactured at scale,” according to the statement.
If successfully approved and authorised, advancing the development of an mRNA-based mpox vaccine candidate could help provide larger supplies of vaccines for use against future mpox outbreaks, the company stated.
The CEPI will provide funding of up to $90 million to support the development of mRNA-based vaccine candidates.
“The strategic partnership between BioNTech and CEPI is aiming to contribute to CEPI’s 100 Days Mission,” according to the statement.
Potential pandemic
The mission’s global goal is to accelerate the development of well-tolerated and effective vaccines against a potential future pandemic virus.
It intends to keep a vaccine ready for regulatory authorization and manufacturing at scale within 100 days of recognition of a pandemic pathogen.
The data generated could contribute to the rapid development of mRNA-based vaccines against future outbreaks caused by orthopoxviruses.
“Mpox can cause severe complications, particularly in children and pregnant women as well as in immunocompromised individuals.,”said Prof. Ugur Sahin, CEO and Co-founder of BioNTech.
“The global outbreak, which was declared a public health emergency of international concern, underlines the need for a highly effective, well-tolerated, and accessible mpox vaccine.”
High medical need
The company’s BNT166 program started in May 2022 and “we believe our scientific approach as well as our mRNA technology have the potential to significantly contribute to deliver on CEPI’s 100 Days Mission,” Prof Sahin said.
Richard Hatchett, Chief Executive Officer of CEPI said: “Our work on mpox could broaden the portfolio of vaccines available against this potentially deadly disease.
It will build “our understanding of how mRNA technology performs against orthopoxviruses, a family of viruses that have long afflicted humankind and remain an ongoing threat today,” he said.
The early clinical trial will evaluate the safety, tolerability, reactogenicity and immunogenicity of two mRNA-based multivalent vaccine candidates for active immunization against mpox.
It is a part of BioNTech’s infectious disease programs aiming to provide equitable access to effective and well-tolerated vaccines for high medical need indications.
This includes BioNTech’s Malaria and Tuberculosis programs, BNT165 and BNT164, respectively, which are both currently in Phase 1 clinical trials.
African facility
BioNTech’s efforts also include establishing a decentralised and robust end-to-end manufacturing network in Africa to enable scalable production of mRNA-based medicines.
Mpox symptoms include skin rash and mucosal lesions, fever, swollen lymph nodes, headache muscle ache and sore throat.
Human-to-human transmission can occur through physical contact, contaminated objects, or body fluids, including sexual contact.