HQ Team
28 April, 2023: A Lyme disease vaccine is in the pipeline, according to reports from pharma companies.
Moderna has announced that the clinical trials for a Lyme vaccine are promising, and shots against the disease will be available by 2030. The fast track of mRNA vaccines against corona has extended and fast-tracked its usage against other diseases.
The company announced two new mRNA vaccines in development that could prevent Lyme disease, marking the “first application of its mRNA technology to bacterial pathogens.”.
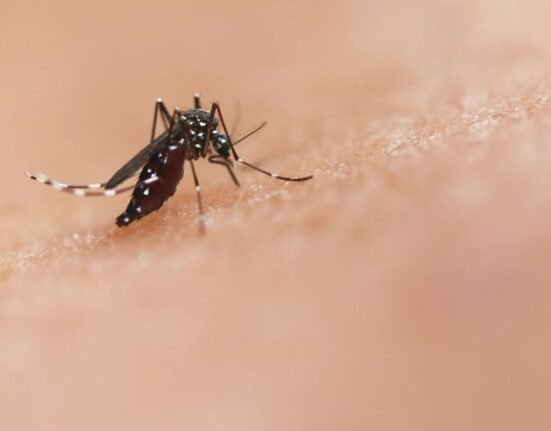
Lyme disease is caused by tick bites and can cause fever, chills, joint pain, and rashes, according to the Centers for Disease Control. If left untreated, it can lead to heart palpitations, arthritis, and facial palsy.
A Lyme disease vaccine, LYMERix, made by the former SmithKline Beecham pharma company, was pulled from the market in 2002 due to low consumer demand and reports of adverse events such as arthritis. Also, anti-vaccine sentiment played a huge part in low sales.
Climate change and Lyme disease
Approximately 120,000 cases of Lyme are reported each year in the U.S. and Europe, and that number is rising due to climate change. “Studies provide evidence that climate change has contributed to the expanded range of ticks, increasing the potential risk of Lyme disease, such as in areas of Canada where the ticks were previously unable to survive,” according to the Environmental Protection Agency. “The life cycle and prevalence of deer ticks are strongly influenced by temperature.”
“Because tick activity depends on temperatures being above a certain minimum, shorter winters could also extend the period when ticks are active each year, increasing the time that humans could be exposed to Lyme disease,” the agency writes.
Continuous fight against Lyme Disease
Other pharma companies that have announced plans to introduce Lyme disease vaccines are Pfizer and its partner company Valneva. The company is hoping to get approvals by 2025. Earlier, its trials were discontinued due to violations of Good Clinical Practice as third-party operators ran its trial sites.
There are other efforts in the works in the fight against the disease. A human monoclonal antibody to be used as pre-exposure prophylaxis (PrEP) for Lyme disease is in the offing. There are plans to inoculate mice in areas with a high incidence of the disease to immunize the ticks carried by the rodents.